![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
196 Cards in this Set
- Front
- Back
|
Acid-base pair |
A pair of two species that transform into each other by gain or loss of a proton. |
|
|
Activation energy |
The minimum energy required to start a reaction by the breaking of bonds. |
|
|
Addition Polymer |
A very long molecular chain, formed by repeated addition reactions of many unsaturated alkene molecules. |
|
|
Addition Reaction |
A reaction in which a reactant is added to an unsaturated molecule to make a saturated molecule. |
|
|
Adsorbtion |
The process that occurs when a gas, liquid or solute is held to the surface of a solid. |
|
|
Alicyclic hydrocarbon |
A hydrocarbon with carbon joining together in a ring structure. |
|
|
Aliphatic hydrocarbon |
A hydrocarbon with carbon atoms joining together in a straight or branched chains. |
|
|
Alkali |
A type of base that dissolves in water to form hydroxide ions, OH⁻ ions. |
|
|
Alkanes |
The homologous with the general formula CnH2n+2 |
|
|
Alkyl group |
An alkane with a hydrogen atom removed. |
|
|
Amount of substance |
The quantity whose unit is the mole. Chemistry use "amount of substance" as a means of counting atoms. |
|
|
Anhydrous |
A substance that contains no water. |
|
|
Anoin |
A negatively charged ion. |
|
|
Average bond enthalpy |
The average enthalpy change that takes place when breaking by homolytic fission 1 mol of a given type of bond in the molecules of a gaseous species. |
|
|
Avogadro's constant |
The number of atoms per mole of the carbon-12 isotope. (6.02x10²³mol⁻¹) |
|
|
Biodegradable substance |
A substance that is broken down naturally in the environment by other living organisms. |
|
|
Biodegradable polymer |
A polymer that breaks down completely into carbon-dioxide and water. |
|
|
Boltzmann distribution |
A diagram showing the distribution of energies of the molecules at a particular temperature. |
|
|
Bond dissociation enthalpy |
The enthalpy change that takes place when breaking by homolytic fission 1mol of a given bond in the molecules of a gaseous species. |
|
|
Brønsted–Lowry acid |
A species that is a proton donor. |
|
|
Brønsted–Lowry base |
A species that is a proton acceptor. |
|
|
Buffer solution |
A system that minimises pH changes on addition of small amounts of an acid or a base. |
|
|
Carbanion |
An organic ion in which a carbon atom has a negative charge. |
|
|
Carbocation |
An organic ion in which a carbon atom has a positive charge. |
|
|
Catalyst |
A substance that increases the rate of a chemical reaction without being used up in the process. |
|
|
Cation |
A positively charged ion. |
|
|
Chemical shift |
A scale that compares the frequency of a NMR absorption with the frequency of the reference peak of TMS at δ=0ppm. |
|
|
Chiral carbon |
A carbon atom attached to four different atoms or groups of atoms. |
|
|
Chromatogram |
A visible record showing the result of separation of the components of a mixture by chromatography. |
|
|
cis-trans isomerism |
A special type of E/Z isomerism in which each carbon of the C=C double bond carries the same atom or group on each side: cis isomerism is the same both sides, trans is different on each side. |
|
|
Complex ion |
A transition metal ion bonded to one or more ligands by coordinate bonds. |
|
|
Condensation Reaction |
A reaction in which two small molecules react together to form one larger molecule with the elimination of a small molecule such as water. |
|
|
Conjugate Acid |
A species formed when a proton is added to a base. |
|
|
Conjugate base |
A species formed when a proton is removed from an acid. |
|
|
Coordinate Bond |
A shared pair of electrons in which the bonded pair has been provided by one bonding atom only, also called a dative covalent bond. |
|
|
Coordinate number |
The total number of coordinate bonds formed between the central metal and any ligands. |
|
|
Cracking |
The breaking down of long-chained saturated hydrocarbons to form a mixture of shorter chained alkanes and alkenes. |
|
|
Curly Arrow |
A symbol used in a reaction to show the movement of a pair of electrons, during the breaking or formation of a covalent bond. |
|
|
Degradable polymer |
A polymer that breaks down into smaller fragments when exposed to light, heat or moisture. |
|
|
Dehydration |
An elimination reaction in which water is removed from a saturated molecule to form an unsaturated molecule. |
|
|
Delocalised electrons |
Electrons that are shared between more that two atoms. |
|
|
Dipole-Dipole Force |
An attractive force between permanent dipoles in neighbouring polar molecules. |
|
|
Displacment reaction |
A reaction in which a more reactive element displaces a less reactive molecule and the bonds between them. |
|
|
Displayed formula |
A formula showing the relative positioning of all the atoms in a molecule and the bonds between them. |
|
|
Disproportionation |
The oxidation and reduction of the same species in a redox reaction. |
|
|
Dynamic equilibrium |
The equilibrium that exists in a closed system when the rate of the forward reaction is equal to the rate of the reverse reaction. |
|
|
E/Z Isomerism |
A type of stereoisomerism in which different groups attached to each carbon of a C=C double bond may be arranged differently in space because of the restricted C=C bond. |
|
|
(First) electron affinity |
The enthalpy change required to add one electron to each atom in one mole of gaseous atoms to form one mole of gaseous 1⁻ ions. |
|
|
Electron Shielding |
The repulsion between electrons in different inner shells. Sheilding reduces the net attractive force from the positive nucleus to an outershell negative electron. |
|
|
Electronic Structure |
The arrangement of electrons in an atom. |
|
|
Electronegativety |
A measure of the attraction of a bonded atom for a pair of electrons in a covalent bond. |
|
|
Electrophile |
An atom which is attracted to an electron-rich centre or atom, where it accepts a pair of electrons to form a new covalent bond. |
|
|
Electrophilic substitution |
A type of substitution reaction in which an electrophile is attracted to an electron-rich centre or atom, where it accepts a pair of electrons to form a new covalent bond. |
|
|
Elimination reaction |
The removal of a molecule from a saturated molecule to make an unsaturated molecule. |
|
|
Empirical formula |
The simplest whole number ratio of atoms of each element present in a compound. |
|
|
Enantiomers |
Stereoisomers that are non-superimposable mirror images of each other, also called optical isomers. |
|
|
Endpoint |
The point in a titration at which there are equal concentrations of the weak acid and conjugate base forms of the indicator. The colour at the end point is midway between the colours of the acid and conjugate base form. |
|
|
Endothermic |
A reaction in which the enthalpy of the products is greater than the enthalpy of the reactants, resulting in heat being taken in from the surroundings (ΔH +ve). |
|
|
(Standard) enthalpy change of atomisation |
The enthalpy change that takes place when one mole of gaseous atoms forms from the element in its standard state. |
|
|
(Standard) enthalpy change of combustion |
The enthalpy change that takes place when one mole of a substance reacts completely with oxygen under standard conditions, all reactants and products being in their standard states. |
|
|
(Standard) enthalpy change of formation |
The enthalpy change that takes place when one mole of a compound is formed from its constituent elements in their standard states under standard conditions. |
|
|
(Standard) enthalpy change of hydration |
The enthalpy change that takes place when one mole of isolated gaseous ions is dissolved in water, forming one mole of aqueous ions, under standard conditions. |
|
|
(Standard) enthalpy change of reaction |
The enthalpy change that accompanies a reaction in the molar quantities expressed in a chemical equation under standard conditions, all reactants and products being in their standard states. |
|
|
(Standard) enthalpy change of solution |
The enthalpy change that takes place when one mole of a compound is completely dissolved in water under standard conditions. |
|
|
Enthalpy cycle |
A diagram showing alternative routes between reactants and products that allows the indirect determination of an enthalpy change from other known enthalpy changes using Hess’s law. |
|
|
Enthalpy profile diagram |
A diagram for a reaction to compares the enthalpy of the reactants with the enthalpy of the products. |
|
|
Enthalpy, H |
The heat content that is stored in a chemical system. |
|
|
Entropy, S |
The quantitative measure of the degree of disorder in a system. |
|
|
Equilibrium law |
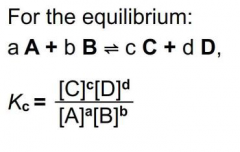
|
|
|
Equivalence point |
The point in a titration at which the volume of one solution has reacted exactly with the volume of the second solution. This matches the stoichiometry of the reaction that is taking place. |
|
|
Esterification |
The reaction of an alcohol with a carboxylic acid to produce an ester and water. |
|
|
Exothermic |
A reaction in which the enthalpy of the products is smaller than the enthalpy of the reactants, resulting in heat loss to the surroundings (ΔH –ve). |
|
|
Fragmentation |
The process in mass spectrometry that causes a positive ion to spilt into pieces, one of which is a positive fragment ion. |
|
|
Free energy change, ΔG |
The balance between enthalpy, entropy and temperature for a process: ΔG = ΔH – TΔS. A process can take place spontaneously when ΔG < 0. |
|
|
Functional group |
The part of the organic molecule responsible for its chemical reactions. |
|
|
General formula |
The simplest algebraic formula of a member of a homologous series. For example, the general formula of the alkanes is CnH2n+2. |
|
|
Giant covalent lattice |
A three-dimensional structure of atoms, bonded together by strong covalent bonds. |
|
|
Giant ionic lattice |
A three-dimensional structure of oppositely charged ions, bonded together by strong ionic bonds. |
|
|
Giant metallic lattice |
A three-dimensional structure of positive ions and delocalised electrons, bonded together by strong metallic bonds. |
|
|
Greenhouse effect |
The process in which the absorption and subsequent emission of infrared radiation by atmospheric gases warms the lower atmosphere and the planet’s surface. |
|
|
Group |
A vertical column in the Periodic Table. Elements in a group have similar chemical properties and their atoms have the same number of outer-shell electrons. |
|
|
Half-life |
The time taken for the concentration of a reactant to reduce by half. |
|
|
Hess's Law |
If a reaction can take place by more than one route and the initial and final conditions are the same, the total enthalpy change is the same for each route. |
|
|
Heterogeneous catalysis |
A reaction in which the catalyst has a different physical state from the reactants; frequently reactants are gases whilst the catalyst is a solid. |
|
|
Heterogeneous equilibrium |
An equilibrium in which the species making up the reactants and products are in different physical states. |
|
|
Heterolytic fission |
The breaking of a covalent bond with both of the bonded electrons going to one atom, forming a cation (+ ion) and an anion (– ion). |
|
|
High-density lipoprotein (HDL) |
A type of lipoprotein that can remove cholesterol from the arteries and transport it back to the liver for excretion or re-utilisation. |
|
|
Homogeneous catalysis |
A reaction in which the catalyst and reactants are in the same physical state, which is most frequently the aqueous or gaseous state. |
|
|
Homogeneous equilibrium |
An equilibrium in which all the species making up the reactants and products are in the same physical state. |
|
|
Homologous series |
A series of organic compounds with the same functional group but with each successive member differing by CH2. |
|
|
Homolytic fission |
The breaking of a covalent bond with one of the bonded electrons going to each atom, forming two radicals. |
|
|
Hydrated |
A crystalline compound containing water molecules. |
|
|
Hydrocarbon |
A compound of hydrogen and carbon only. |
|
|
Hydrogen Bond |
A strong dipole–dipole attraction between an electron-deficient hydrogen atom (O–Hδ+, N–Hδ+ or F–Hδ+) on one molecule and a lone pair of electrons on a highly electronegative atom (HδO:δ–, HδN:δ– HδF:δ–) on a different molecule. |
|
|
Hydrolysis |
A reaction with water that breaks a chemical compound into two compounds; the H and OH in a water molecule becomes incorporated into the two compounds. |
|
|
Initial rate of reaction |
The change in concentration of a reactant or product per unit time at the start of the reaction: t = 0. |
|
|
Initiation |
The first step in a radical substitution in which the free radicals are generated by ultraviolet radiation. |
|
|
Intermediate |
A species formed in one step and used up in a subsequent step and so never seen as either a reactant or a product. |
|
|
Intermolecular force |
An attractive force between neighbouring molecules. Intermolecular forces can be van der Waals’ forces, dipole–dipole forces or hydrogen bonding. |
|
|
Ion |
A positively or negatively charged atom or a (covalently bonded) group of atoms (a molecular ion). |
|
|
Ionic bonding |
The electrostatic attraction between oppositely charged ions. |
|
|
Ionic product of water, Kw |
Kw = [H+(aq)] [OH–(aq)] At 25 °C, Kw = 1.00 × 10–14 mol2 dm–6. |
|
|
(First) ionisation energy |
The energy required to remove one electron from each atom in one mole of gaseous atoms to form one mole of gaseous 1+ ions. |
|
|
(Second) ionisation energy |
The energy required to remove one electron from each ion in one mole of gaseous 1+ ions to form one mole of gaseous 2+ ions. |
|
|
Isoelectric Point |
The point at which an amino acid has no overall charge. |
|
|
Isotopes |
Atoms of the same element with different numbers of neutrons and different masses. |
|
|
Lattice enthalpy |
The enthalpy change that accompanies the formation of one mole of an ionic compound from its gaseous ions under standard conditions. |
|
|
Le Chatelier’s Principle |
When a system in dynamic equilibrium is subjected to a change, the system readjusts itself to minimise the effect of the change and to restore equilibrium. |
|
|
Ligand |
A molecule or ion that can donate a pair of electrons to a transition metal ion. |
|
|
Ligand substitution |
A reaction in which one ligand in a complex ion is replaced by another ligand. |
|
|
Limiting reagent |
The substance in a chemical reaction that runs out first. |
|
|
Lone pair |
An outer-shell pair of electrons that is not involved in chemical bonding. |
|
|
Low-density lipoprotein (LDL) |
A type of lipoprotein responsible for carrying cholesterol and triglycerides from the liver to the tissues. |
|
|
Mass (nucleon) number |
The number of particles, protons and neutrons, in the nucleus. |
|
|
Mechanism |
A sequence of steps, showing the path taken by electrons in a reaction. |
|
|
Metallic bond |
The electrostatic attraction between positive metal ions and delocalised electrons. |
|
|
Mobile phase |
The phase that moves in chromatography. |
|
|
Molar mass, M |
The mass per mole of a substance. The units of molar mass are g mol–1. |
|
|
Mole |
The amount of any substance containing as many elementary particles as there are carbon atoms in exactly 12 g of the carbon-12 isotope. |
|
|
Molecular formula |
The actual number of atoms of each element in a molecule. |
|
|
Molecular ion, M+ |
The positive ion formed in mass spectrometry when a molecule loses an electron. |
|
|
Molecule |
A small group of atoms held together by covalent bonds. |
|
|
Monomer |
A small molecule that combines with many other monomers to form a polymer. |
|
|
Neutralisation |
A chemical reaction in which an acid and a base react together to produce a salt and water. |
|
|
Nomenclature |
A system of naming compounds. |
|
|
Nucleophile |
An atom (or group of atoms) which is attracted to an electron-deficient centre or atom, where it donates a pair of electrons to form a new covalent bond. |
|
|
Order |
The power to which the concentration of the reactant is raised in the rate equation. |
|
|
Overall order |
The sum of the individual orders: m + n. |
|
|
Oxidation |
Loss of electrons or an increase in oxidation number. |
|
|
Oxidation number |
A measure of the number of electrons that an atom uses to bond with atoms of another element. Oxidation numbers are derived from a set of rules. |
|
|
Oxidising agent |
A reagent that oxidises (takes electrons from) another species. |
|
|
Peptide |
A compound containing amino acids linked by peptide bonds. Often the number of amino acids is indicated by the prefix, di-, tri-, tetra-: |
|
|
Percentage yeild |
(actual amount / theoretical amount) x 100 |
|
|
Period |
A horizontal row of elements in the Periodic Table. Elements show trends in properties across a period. |
|
|
Periodicity |
A regular periodic variation of properties of elements with atomic number and position in the Periodic Table. |
|
|
Permanent dipole |
A small charge difference across a bond resulting from a difference in electronegativities of the bonded atoms. |
|
|
pH |
pH = –log[H^+(aq)] [H^+(aq)] = 10^–pH. |
|
|
Pharmacological activity |
The beneficial or adverse effects of a drug on living matter. |
|
|
Phase |
A physically distinctive form of a substance, such as the solid, liquid and gaseous states of ordinary matter. |
|
|
pi-bond |
The reactive part of a double bond formed above and below the plane of the bonded atoms by sideways overlap of p-orbitals. |
|
|
Polar covalent bond |
A bond with a permanent dipole. |
|
|
Polar molecule |
A molecule with an overall dipole, having taken into account any dipoles across bonds. |
|
|
Polymer |
A long molecular chain built up from monomer units. |
|
|
Precipitation reaction |
The formation of a solid from a solution during a chemical reaction. Precipitates are often formed when two aqueous solutions are mixed together. |
|
|
Principal quantum number, n |
A number representing the relative overall energy of each orbital, which increases with distance from the nucleus. The sets of orbitals with the same n-value are referred to as electron shells or energy levels. |
|
|
Propagation |
The two repeated steps in radical substitution which build up the products in a chain reaction. |
|
|
Radical |
A species with an unpaired electron. |
|
|
Rate constant, k |
The constant that links the rate of reaction with the concentrations of the reactants raised to the powers of their orders in the rate equation. |
|
|
Rate equation |
For a reaction: A + B → C, the rate equation is given by: |
|
|
Rate of Reaction |
The change in concentration of a reactant or a product in a given time. |
|
|
Rate-determining step |
The slowest step in the reaction mechanism of a multi-step reaction. |
|
|
Reaction mechanism |
A series of steps that, together, make up the overall reaction. |
|
|
Redox reaction |
A reaction in which both reduction and oxidation take place. |
|
|
Reducing agent |
A reagent that reduces (adds electrons to) another species. |
|
|
Reduction |
Gain of electrons or a decrease in oxidation number. |
|
|
Reflux |
The continuous boiling and condensing of a reaction mixture to ensure that the reaction takes place without the contents of the flask boiling dry. |
|
|
Relative atomic mass, Ar |
The weighted mean mass of an atom of an element compared with one-twelfth of the mass of an atom of carbon-12. |
|
|
Relative formula mass |
The weighted mean mass of the formula unit of a compound compared with one-twelfth of the mass of an atom of carbon-12. |
|
|
Relative isotopic mass |
The mass of an atom of an isotope compared with one-twelfth of the mass of an atom of carbon-12. |
|
|
Relative molecular mass, Mr |
The weighted mean mass of a molecule of a compound compared with one-twelfth of the mass of an atom of carbon-12. |
|
|
Repeat unit |
A specific arrangement of atoms that occurs in the structure over and over again. Repeat units are included in brackets, outside which is the symbol n. |
|
|
Retention time |
The time for a component to pass from the column inlet to the detector. |
|
|
Rf value |

|
|
|
Salt |
A chemical compound formed from an acid, when an H+ ion from the acid has been replaced by a metal ion or another positive ion, such as the ammonium ion, NH4+. |
|
|
Saturated hydrocarbon |
A hydrocarbon with single bonds only. |
|
|
Shell |
A group of atomic orbitals with the same principal quantum number, n. Also known as a main energy level. |
|
|
Simple molecular lattice |
A three-dimensional structure of molecules, bonded together by weak intermolecular forces. |
|
|
Skeletal formula |
A simplified organic formula, with hydrogen atoms removed from alkyl chains, leaving just a carbon skeleton and associated functional groups. |
|
|
Specific heat capacity, c |
The energy required to raise the temperature of 1 g of a substance by 1 °C. |
|
|
Spectator ions |
Ions that are present but play no part in a chemical reaction |
|
|
Spin–spin coupling |
The interaction between spin states of non-equivalent nuclei that results in a group of peaks in an NMR spectrum. |
|
|
Stability constant, K stab |
The equilibrium constant for an equilibrium existing between a transition metal ion surrounded by water ligands and the complex formed when the same ion has undergone a ligand substitution reaction. |
|
|
Standard conditions |
A pressure of 100 kPa (1 atmosphere), a stated temperature, usually 298 K (25 °C) and a concentration of 1 mol dm–3 (for reactions with aqueous solutions). |
|
|
Standard electrode potential, |
The e.m.f. of a half cell compared with a standard hydrogen half cell, measured at 298 K with solution concentrations of 1 mol dm–3 and a gas pressure of 101 kPa (1 atmosphere). |
|
|
Stationary phase |
The phase that does not move in chromatography. |
|
|
Stem |
The longest carbon chain present in an organic molecule. |
|
|
Stereoisomers |
Compounds with the same structural formula but with a different arrangement of the atoms in space. |
|
|
Stoichiometry |
The molar relationship between the relative quantities of substances taking part in a reaction. |
|
|
Stratosphere |
The second layer of the Earth’s atmosphere, containing the ‘ozone layer’, between about 10 km and 50 km above the Earth’s surface. |
|
|
Strong acid |
An acid that completely dissociates in solution. |
|
|
Structural formula |
A formula showing the minimal detail for the arrangement of atoms in a molecule. |
|
|
Structural isomers |
Molecules with the same molecular formula but with different structural arrangements of atoms. |
|
|
Sub-shell |
A group of the same type of atomic orbitals (s, p, d or f) within a shell. |
|
|
Substitution reaction |
A reaction in which an atom or group of atoms is replaced with a different atom or group of atoms. |
|
|
Suffix |
The part of the name added after the stem. |
|
|
Termination |
The step at the end of a radical substitution when two radicals combine to form a molecule. |
|
|
Thermal decomposition |
The breaking up of a chemical substance with heat into at least two chemical substances. |
|
|
Transition element |
A d-block element which forms an ion with an incomplete d sub-shell. |
|
|
Troposphere |
The lowest layer of the Earth’s atmosphere, extending from the Earth’s surface up to about 7 km (above the poles) to about 20 km (above the tropics). |
|
|
Unsaturated hydrocarbon |
A hydrocarbon containing multiple carbon-to-carbon bonds. |
|
|
Valence shell |
The outermost shell of an atom, which contains the electrons most likely to react and bond to other atoms. |
|
|
van der Waals’ force |
An attractive force between instantaneous dipoles and induced dipoles in neighbouring molecules. |
|
|
Volatility |
The ease with which a liquid turns into a gas. Volatility increases as boiling point decreases. |
|
|
Water of crystillisation |
Water molecules that form an essential part of the crystalline structure of a compound. |
|
|
Weak acid |
An acid that partially dissociates in solution. |
|
|
Zwitterion |
A dipolar ionic form of an amino acid that is formed by the donation of a hydrogen ion from the carboxyl group to the amino group. As both charges are present there is no overall charge. |

