![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
Properties of metals |
Solid at room temperature Iron, Nickel and Cobalt are magnetic Conducts electricity and heat Mercury is a liquid |
|
|
What do the words DUCTILE, SONOROUS AND MALLEABLE mean? |
Ductile=can be drawn into a wire Sonorous=makes a sound when struck Malleable=can bend and shape easily |
|
|
What are these things used for. Aluminum, Copper, Gold, Iron, and Mercury? |
Aluminum=low density-aircraft parts Copper=electrical conductor-electrical wires Iron=very strong=bridges and cars, high tensile strength Mercury=liquid at room temperature- thermometers |
|
|
What is Tarnishing? |

Tarnishing occurs when a metal loses its shine. Metals react with air and water which = thin layer of Tarnish. IRON+OXYGEN+ WATER----IRON OXIDE |
|
|
How to stop rusting. |
Galvanishing= when a layer of Zinc stops the oxygen and water from hitting the metal. Painting and Greasing= stops oxygen and water reaching the metals. Electroplating. |
|
|
Group 1 Elements Periods go across and groups go downwards? |
LITHIUM-Li SODIUM-So POTASSIUM-K RUBIDIUM- CAESIUM- FRANCIUM- |
|
|
Equations: |
Magnesium+oxygen--magnesium oxide Zinc+hydrochloric acid--zinc chloride+hydrogen Sodium+water--sodium hydroxide+hydrogen Magnesium+sulfuric acid--magnesium sulfate+hydrogen |
|
|
State Symbols: |
SOLID-S LIQUID-L GAS-G AQUEOUS SOLUTION-AQ |
|
|
Metal carbonates and acids. General equation: |
GENERAL EQUATION: Metal carbonate+acid--salt+carbon dioxide+ water e.g. Calcium carbonate+hydrochloric acid--calcium chloride+carbon dioxide+water Zinc carbonate+hydrochloric acid--zinc chloride+carbon dioxide+water |
|
|
Periodic Table: |
Nitrogen-N Zinc-Zn Oxygen-O Sodium-So Iron-Fe Hydrogen-H Calcium-Ca Chlorine-Cl Magnesium-Mg Aluminum-Al Potassium-K Cobalt-Co Nickel-Ni |
|
|
BALANCING QUESTION: |
Magnesium+hydrochloric acid--magnesium chloride+hydrogen Mg + 2HCl-- MgHCl2+ H2 |
|
|
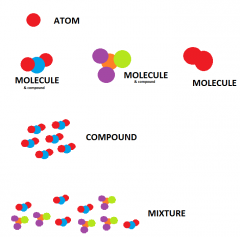
What is an Element, Compound, Mixture, Atom, molecule? |
Element=substance made up from just one type of atom Atom=single, very small particle Mixture=substance made of atoms/molecules, compounds, not chemically bonded together. Compound=substance made of one or two or more different elements, chemically bonded together |
|
|
ELEMENT COMPOUND !!!!!!PICTURES!!!! MIXTURE ATOM MOLECULE |
!LEARN OF BY HEART! |

