![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
80 Cards in this Set
- Front
- Back
|
Ionic bonding |
• Ionic bonding is when an element (non-metal or metal) loses or gains an electron in order to fill their outer shell. • Metals lose electrons to form cations (positive ions) and non-metals gain electrons to form anions (negative ions). • An example is NaCl. |
|
|
Isotopes |
Different forms of the same atoms with the same number of protons but a different number of neutrons. |
|
|
Covalent bonding |
• Covalent bonding is when two or more non-metals share electrons in order to complete their outer shells. • An example is H20. |
|
|
Alloys |
A mixture of two or more elements, one of which is a metal. |
|
|
Relative mass number |
(Mass number of isotope 1 x percentage) + (mass number of isotope 2 x percentage) / 100 |
|
|
Relative formula mass |
Multiply each elements atomic mass by number of it present in compound then add numbers together. |
|
|
Calculating moles |
Mass in grams / formula mass |
|
|
Calculating the percentage composition of an element |
No of atoms of element x relative atomic mass / relative formula mass x 100 |
|
|
Calculating empirical formula |
1) list elements in compound and write percentage or given mass 2) divide by relative atomic mass 3) turn into ratio by multiplying/dividing by a well chosen number 4) get into simplest form ratio |
|
|
Calculating the mass in reactions |
1) write balanced equation 2) work out relative formula mass 3) divide to get 1g and multiply to get amount of vs asked in question |
|
|
Percentage yield |
Actual yield / theoretical yield |
|
|
Properties of metallic bonding (4 things)
|
• electrostatic bond between positive neutrons and de-localised electrons in metals. • enables metals to be good conductors of electricity because lots of freely moving electrons and enables metals. •enables metals to be malleable because the de-localised electrons are always moving around ensuring there is an attraction between the positive neutrons and the negative electrons which means they won’t repel and the structure doesn't fall apart but the layers can slide over each other. • strong electrostatic forces between the opposite charges leads to high melting and boiling points. |
|
|
Properties of diamond (4 things)
|
• Made up of carbon atoms. • Atoms covalently bonded to 4 other carbon atoms • Strong covalent bonds make it hard. • Doesn't conduct electricity because there are no free electrons or ions to carry the charge through the substance . |
|
|
Properties of graphite (3 things)
|
•Atoms covalently bond to three other carbon atoms forming layers. • No covalent bonds between layers meaning they can slide over each other - soft and suitable for pencils. • Conducts electricity because delocalised electrons can move within layers and carry electrical charges through the substance. |
|
|
Properties of silica (3 things)
|
• Made up of silicon and oxygen atoms covalently bonded. • Very hard because of covalent bonds. • Poor conductor of electricity because there are no free electrons or ions to carry the charge through the substance. |
|
|
Properties of Giant Covalent Substances (3 things).
|
•High melting and boiling points - in order to boil or melt it, the atoms would have to be pulled apart, hard because atoms all connected by strong covalent bonds which needs a lot of energy to break them up and this comes from heat. •Unreactive chemically. •Hard. |
|
|
Properties of Giant Ionic Substances (3 things).
|
• High melting and boiling points - in order to melt, ions have to be pulled apart and electrostatic forces have to be broken. electrostatic forces very strong so a lot of energy needed to break them up. •Have a regular lattice structure as very strong electrostatic forces of attraction act in all directions. • When the ions in giant ionic substances are held in place the ions cannot move as they are strongly attracted to each other so they cannot carry a charge through the substance but when it is molten or dissolved in water then the ions are free to move as the attraction between them is not as strong and therefore can conduct electricity. |
|
|
Properties of Simple Molecular Substances (3 things).
|
• Have low melting and boiling points - need little energy to break up weak intermolecular forces. • Can't conduct electricity because molecules have no overall electric charge. • Gases or liquids at room temperature due to their low melting and boiling points. |
|
|
Simple Molecular Substance
|
Simple molecular substances are made up from molecules (a collection of atoms joined up by covalent bonds). Examples include CO2, H2O and O2.
|
|
|
Properties of alloys
|
Because of the different sizes of metals, the layers are distorted which make it more difficult for them to slide over each other. This makes alloys harder than pure metals.
|
|
|
Properties of thermosoftening plastics
|
Soften when heated because weak intermolecular forces in the polymer chain are broken down when it is heated and it becomes soft. When the polymer cools, the intermolecular forces bring the molecules back together and the polymer hardens.
|
|
|
Thermosoftening plastics
|
Plastics that soften and can be reshaped and remoulded when heated.
|
|
|
Properties of thermosetting plastics
|
Can't soften when heated because strong covalent bonds form cross-links between their polymer chains and hold them in position.
|
|
|
Thermosetting plastics
|
Plastics that don't soften and can't be reshaped when heated.
|
|
|
Nanoscience
|
small particles between 1-100 nanometres in size.
|
|
|
Nanotechnology
|
Uses nanoparticles as catalysts and cosmetics such as sun cream and deodorants.
|
|
|
Reversible reactions
|
When the products of the reaction can react to make the original reactants. E.g. Ammonium Chloride. In one direction the reaction is endothermic and in the other, the reaction is exothermic.
|
|
|
Why it is not possible to get 100% yield from a reaction
|
• Reactions may not go to completion. • Other unwanted reactions may occur. • Products may be lost when it is separated or collected from the apparatus. |
|
|
Paper chromatography
|
• Used to analyse artificial colours in food. • Spot of colour is put on paper. • Solvent allowed to move through the paper. • Colours move different distances depending on solubility. |
|
|
Advantages of instrumental analysis (4 things)
|
•Rapid. •Accurate. •Sensitive. •Useful when using small samples. |
|
|
Disadvantages of instrumental analysis
|
•Expensive. •requires special training to be used. |
|
|
Gas chromatography
|
• Used to separate substances in a mixture so that they can be identified. • Mixture carried by gas through long column packed with particles of an inert solid. • Individual compounds in mixture travel at different speeds through column and come out at different times. • Time at which a substance leaves the column is recorded (retention time). • Mass spectrometer identifies which substance an individual compound is by comparing its retention time to already known substances. |
|
|
Rate of reaction
|
Amount of reactant used/time or amount of product formed/ time.
|
|
|
Collision theory
|
States that particles must collide with a certain amount of energy (activation energy) before they can react.
|
|
|
Factors which increase chance of collision
|
- Increasing temperature. - Increasing the concentration of a solution. - Increasing the pressure of gases. - Increasing the surface area of solids. - Using a catalyst. |
|
|
The effect of temperature on a reaction.
|
When the temperature increases, the particles have more energy and collide more often (increasing the chance of successful collisions) and harder, meaning the rate of reaction increases.
|
|
|
The effect of concentration or pressure on a reaction.
|
When the concentration or pressure increases, the frequency of collisions increases, which means the rate of reaction increases.
|
|
|
The effect of surface area on a reaction.
|
The larger the surface area, the more the particles are exposed to the other reactant and there is an increased chance of collision, therefore meaning the rate of reaction increases.
|
|
|
Catalyst
|
Speeds up the rate of reaction without being used up during the reaction by providing an alternative route and lowering the activation energy.
|
|
|
Exothermic reactions
|
Reactions which give out energy.
|
|
|
Endothermic reactions
|
Reactions which take in energy from the surroundings.
|
|
|
Examples of Exothermic reactions
|
Combustion, oxidation reactions such as respiration and neutralisation reactions.
|
|
|
Examples of Endothermic reactions
|
Decomposition, electrolysis.
|
|
|
Uses of Exothermic reactions
|
Hand warmers, self-heating cans.
|
|
|
Uses of Endothermic reactions
|
Instant cold packs.
|
|
|
Acid (2 things)
|
• Substances that produce H+ ions when added to water. • pH value of 0 – 6. |
|
|
Alkali (2 things)
|
• Substances that produce OH- ions when added to water. • pH value of 8 - 14. |
|
|
Base (2 things)
|
• A substance which neutralises an acid and is a metal oxide, hydroxide or carbonate. • All soluble bases are alkalis. |
|
|
Indicator
|
Dyes that change colour in acids and alkalis.
|
|
|
Solubility rules
|
All Sulphates are soluble apart from silver, barium, calcium and lead Sulphates. All Chlorides are soluble apart from silver and Lead Chlorides. All Hydroxides and Carbonates are insoluble. SNAP - Sodium, Nitrates, Ammonium and Potassium are all soluble. |
|
|
Precipitation
|
Formation of insoluble salts by reacting two aqueous, colourless solutions.
|
|
|
How to make salts when reacting an acid with an alkali (titration)
|
• Fill burette with acid. Empty jet at bottom of burette. • Fill pipette with solution. Let it run out into conical flask. • Add drops of indicator to conical flask to change colour. • Let acid run out little at time to conical flask. Swirl flask. • When solution changes colour to colourless, slow down acid so it is dripping drop by drop until one drop changes colour permanently. • Pour solution from conical flask into evaporating dish and evaporate off water. Allow to cool so crystals form. |
|
|
Electrolysis
|
process of separating metals above Carbon in the reactivity series from its ionic compound using electricity.
|
|
|
O I L R I G |
Oxidation is loss of electrons, reduction is gain of electrons. |
|
|
Cation
|
Positively charged ion. |
|
|
Anion
|
Negatively charged ion.
|
|
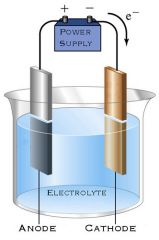
AA and CC
|
Anode (positively charged electrode) attracts anions. Cathode (negatively charged electrode) attracts cations.
|
|
|
What change occurs at the Anode?
|
Oxidation occurs at the Anode as the negative ions lose electrons. When aqueous solutions are electrolysed, Oxygen is produced at the Anode unless Halide ions are present. |
|
|
What change occurs at the Cathode?
|
Reduction occurs at the Cathode as the positive ions gain electrons. When aqueous solutions are electrolysed, Hydrogen is produced at the Cathode unless ions in solution belong to metals above Hydrogen. |
|
|
What is electroplating and why is it done?
|
Uses electrolysis to put a thin coating of metal onto a metal in order to: - make an object more attractive. - protect an object from corroding. - increase the hardness of an area. - reduce costs by using a thin layer of metal instead of pure metal. |
|
|
Example: Copper plating
|
• Copper at Anode is an atom, loses electrons and falls into solution as Cu2+. • Electrons go to Cathode and are picked up by Cu2+ ions in the electrolyte which produces copper again. |
|
|
Rules of electroplating
|
- Anode is object to be plated e.g. impure Copper in Copper plating. - Cathode is plating metal e.g. pure Copper in Copper plating. - Electrolyte contains ions of the plating metal e.g. Copper Sulphate in Copper Plating |
|
|
Compound
|
a substance that consists of two or ore elements which are chemically combined together.
|
|
|
What are fullerenes and what are they used for?
|
Hexagonal rings of carbon atoms that can be used for: •drug delivery into the body. •lubricants. •catalysts. •reinforcing materials. |
|
|
Shape memory alloys (with examples)
|
alloys that when cooled can be bent and reshaped and heated to go back to original shape. Example nitinol used for dental braces. |
|
|
The conditions in which a polymer is made affects its properties . This is evident with: (name and state conditions made and uses)
|
•LD - heating ethene to 200 degrees under high pressure. used for plastic bags and bottles. •HD - heating ethene at low temperatures and pressure with a catalyst. used for water tanks and drainpipes. |
|
|
mass number
|
number of protons and neutrons.
|
|
|
atomic number
|
number of protons (= electrons).
|
|
|
Activation energy
|
minimum amount of energy particles must have in order for there to be a reaction.
|
|
|
Positives of using catalysts
|
•saves money as production doesn't need to operate for as long. •allows some productions to be done at lower temperatures reducing the energy used up. •never used up so can be used again. |
|
|
Negatives of using catalysts
|
•Expensive. •different reactions use different catalysts. •can be poisoned by impurities which means you have to keep the reaction very clean. |
|
|
what two products are produced when an acid and a metal are reacted?
|
salt and hydrogen.
|
|
|
what two products are produced when an acid and an alkali are reacted?
|
salt and water.
|
|
|
How to make an insoluble salt (precipitation)
|
•mix solutions with ions needed preset. •precipitate formed in bottom of flask - filter, dry and wash. |
|
|
How to make a soluble salt with metals/insoluble bases
|
•add metal/insoluble base to acid. •solid dissolves in acid and excess metal sinks to bottom of flask. •filter, evaporate water and leave to form crystals. |
|
|
What are ammonium salts used for?
|
fertilisers.
|
|
|
Electrolysis of aluminium oxide
|
•cryolite lowers melting point of aluminium oxide to 900 degrees. •electrodes made of carbon (as its a good conductor of electricity). •aluminium formed at cathode, oxygen formed at anode. •oxygen reacts with carbon so electrodes need to be replaced. |
|
|
What is produced in the electrolysis of sodium chlorine?
|
hydrogen, chlorine, sodium hydroxide.
|
|
|
If halides and hydroxides are present, what is formed in electrolysis?
|
halides.
|
|
|
If a metal in electrolysis is more reactive than hydrogen what is formed?
|
hydrogen.
|

