![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
52 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
4 fundamental propeties of a gas? |
Mass, volume, temperature, pressure |
|
|
|
What is STP? |
standard temperature and pressure temperature =0 degrees Pressure = 1atm (101.33kpa = 760mmHg = 760 torr) |
|
|
|
the volume occupied by 1 mole of any gas at STP is refered to as?
is equal to? |
Standard molar volume
equal to 22.4L |
|
|
|
what does kinetic molecular theory describe? |
behaviour of particles in a gas an "ideal gas" fits this theory |
|
|
|
what are the essential points of kinetic molecular theory of gasses? |
gasses = extremely small molecules or atoms massively spaced apart, relative to particle size 2) particles in gas are in constant motion, except when they collide 3) particles of an ideal gas exert no attractive or repulsive force on one another 4) collisions of particles does not, on average, slow them down; they cause a change in the direction of the particles. collisions result in transfer of energy between particles (total energy is constant) 5) average kinetic energy (KE = 1/2 mv^2) increases with temperature. (KE = 3/2kT) temperature is measured on kelvin scale.* k=the boltzmann constant typical speed of a gas particle is directly proportional to the square root of the absolute temperature |
*kelvin scale is an absolute scale. it starts at absolute 0 |
|
|
at a certain temperature, the particles of a gas will assume a specific distribution of kinetic energies. if temperature is increased, what happens to this distribution of kinetic energies? |

more particles will posess the minimum escape KE the peak of the distribution curve will flatten, and move left. meaning larger range of KEs, and particles have a higher kinetic energy overall. this plot shows distribution of collision energies of individual particles similar distribution to liquid particles. (figure actually refers to liquds, as there is the escape KE) |
|
|
|
what 4 propeties of a gas can we measure experimentally? |
Weight. from which we can calculate N (number of molecules/atoms) Pressure (P) of gas exerted on the walls of the container it's in volume (V) Temperature (T) if we know any 3, we can calculate the 4th. 3 is the minimum number of propeties we need to describe the state of an ideal gas |
|
|
|
Grahms law describes? |
the mean free path of any gas particle taken per unit volume process = diffusion. effusion = related |
|
|
|
Diffusion is? |

flow of gas particles spreading out evenly through random motion. [high] to [low] inversely proportional to the square root of their respective molar masses. (equation) rate 1/Rate 2 = diffusion rate for gas 1 and gas 2 M1/M2 = molar masses of gases 1 and 2 |
|
|
|
effusion is? |

movement of a gas through a small hole of pore into another gaseous region/vacuum. if pore is large enough, it is diffusion. rate of effusion is the same as diffusion: the ratio of effusion rates (rate 1 / rate 2) is inversely proportional to the square root of their molar masses. |
|
|
|
Charles law is? |
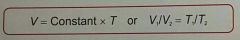
V (volume) of a gas is directly proportional to the absolute temperature (kelvin), when P and N are constant (Pressure and number of moles/particles.) |
|
|
|
Boyle's law is? |

the volume (V) of a fixed weight of gas held at constant temperature varies inversely with pressure. basically, more pressure = smaller gas (duhhhhhh) |
|
|
|
What is avagadro's law? |

Volume of a gas at a constant temperature and pressure is directly proportional to the number of particles or moles (n) of the gas present |
|
|
|
Combined gas law is? |

for a given constant mass of any gas:
The product of it's pressure and volume divided by its kelvin temperature is equal to a constant (k)
use the constant gas law to calculate any of the 3 variables of a gas exposed to 2 seperate conditions: [image]
a change in pressure, volume and/or temperature will be affected as a function of the other quantities (P2, V2, T2) |
|
|
|
The ideal gas law is? |

combination of boyle's law, charles's law and avogadro's law. R is the universal gas constant = 0.0921 L-atm/K-mole = 8.31 kPa-dm^3/K-mole |

|
|
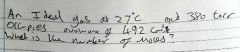
|

convert to the units of the R gas constant (kelvin, litres, atm) - ( R could also be given in kpa-dm^3-kelvin) these problems often are assesing your ability to convert units. |

|
|
|
in terms of PV=, how else might the ideal gas law be given/ |
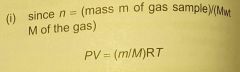
|
|
|
|
what is the density of gas? in terms of P (pressure), what does P = (ideal gas law) |
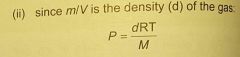
d=m/v |
|
|
|
in a mixture of gases, do all particles excert an equal pressure on the walls of a container? |
yes. if you have more Na+ than k, then each particle is exerting the same pressure, but you have more Na particles exerting the same pressure. meaning that pressure of Na is collectively higher. more Na particles are vying to escape. |
|
|
|
partial pressure refers to? |
the pressure of one of the components (particle species) from the mixture of a gas occupying a volume and temperature partial pressure gives the pressure if that component was isolated in the same volume and temperature. |
|
|
|
Daltons law states? |
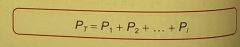
total pressure of a mixture of gasses = sum of pressures that each individual component would exert if it were alone in the container. total pressures = sum of partial pressures |
|
|
|
what is the mole fraction (Xi) for any one particle species in a gas/. |
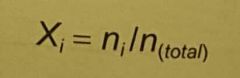
individual atom moles = number of moles of individual atom / total number of moles of particles the sum of all mole fractions must = 1 (obviously) (hint) |
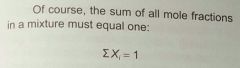
|
|
|
how do you work out partial pressure? |
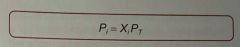
partial pressure = mole fraction of that particle species / total pressure simple. ideal gas law applies to partial pressures (components in a mixture) PiV = niRT [subscript i] |
|
|
|
What is a Real gas? how does it differ from an ideal gas? |
a gas that has deviated from the ideal gas. it no longer obeys the ideal gas law PV=nRT particles experience intermolecular forces (in an ideal gas, there are no intermolecular forces.) - e.g. van der waal forces deviations caused by intermolecular forces are more pronounced at lower T. (although independent of T) increase in pressure = more crowded gas = reduced distance between particles = increased attractive force between particles |
|
|
|
What causes a particle to deviate from an ideal gas to a real gas? |
high pressure, low temperature ideal gas exists when pressure is low and temperature is high = "an ideal Plow and Thigh" |
|
|
|
At a given temperature, if a real gas is subjected to high pressure, the volume the gas takes up is what, compared with the volume expected by the ideal gas law? |
smaller volume than that expected. particles = closer together. =more chance of intermolecular attraction this intermolecular attraction exerts a second force, making the volume a bit smaller than expected. deviates appreciably from the ideal gas law , which doesn't take these intermolecular forces into account. |
|
|
|
A larger particle will deviate how much from ideal gas law behaviour compared with a smaller particle. and why? (at the same KE) |
larger particle with the same KE as a smaller particle moves slower than the smaller particle attractive forces on heavier particles = greater, as they are slower. = larger deviation from ideal gas law smaller particles = greater speed, = counteracting attractive forces = smaller deviation from ideal gas law |
(KE=1/2mv^2) |
|
|
how does temperature affect intermolecular attraction? |
low temperature = decreased average velocity of particles = higher significance of intermolecular forces
= smaller volume than would be predicted by the ideal gas law |
|
|
|
degree to which 2 liquids can mix is called? |
miscibility |
|
|
|
propeties of liquids |
viscosity. surface tension incompressible cohesion (intermolecular attraction) adhesion (attraction to their surroundings) hydrophobic surface = no adhesion. |
|
|
|
Name the van der walls forces |
dipole-dipole forces london forces dipole-induced dipole forces hydrogen bonds these forces are weak. they are only effective over short distances (low temperatures) methane = non-polar. = weak intermolecular forces. |
|
|
|
describe Dipole-dipole forces and dipole-induced dipole forces |
dipole-dipole depend on orientation and distance between molecules. inversely proportional to the 4th power of the distance dipole-induced dipole is inversely proportional to the 7th power of the distance. relatively independent of orientation a dipolar molecule (permanently dipolar) induces a dipole in a neighboring molecule |
|
|
|
Describe London forces |

London forces = dispersive forces. attractive forces between non-polar molecules due to unsymettrical INSTANTANEOUS electron distribution which induces a dipole in a neighboring molecule. leading to attractive force. the dipoles formed are short lived in their lifetime, they interact with neighboring molecules, inducing more dipoles London (dispersion) forces are responsible for the liquefaction of noble gases at low temperatures (and high pressures) |
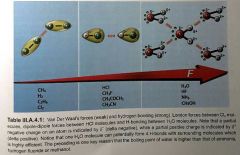
|
|
|
Hydrogen bonds are? |
when H is bonded to an atom such as O, N or F, electrons are unevenly distributed O is more electronegative than H, so O gets a greater share of electrons the electrons sharing the bond = closer to the O nucleus H=relatively de-shelled. H now = strongly attracted to O, N or F atoms of neighboring molecules, singe O (N or F) forms the negative end of a strong dipole --- so the slightly positive charge of H is attracted to slightly MORE electronegative atoms of nearby molecules H bonds are dipole-dipole bonds H bonds are the strongest intermolecular force Not as strong as intramolecular bonds obv. charactised by unusually strong interactions and high boiling points (due to vast amount of energy required to break them) H-bond atoms = FON [and a little bit sulphur, but to a far weaker extent - FON are more electronegative]
|
|
|
|
Viscosity is? |

the friction between moving liquids results in dissipation of mechanical energy: as one layer flows over another, it's motion is transmitted to the second layer. the second layer is now set in motion from this. energy from the first layer is lost to the second layer in this transfer of momentum a larger transfer of momentum = more energy lost from each layer = slower movement = higher viscosity viscosity coefficient (image) measures efficiency of transfer of momentum. high {n - image] = high viscosity. = large loss of mechanical energy. = slow flowing (molasses) low [image, n] = e.g. water |
|
|
|
name a crystaline solid and an amorphous solid |
crystaline: NaCl - structure with ordered geometric shape. high melting point amorphous: molecular structure with no specific shape. melts over a wide range of temperatures since molecules require differing energies to break the bonds between them |
|
|
|
In phase equilibria, what is a phase
example of a phase change? |
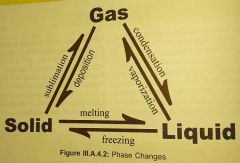
homogenous, physically distinct, mechancally seperable
ice/liquid water / water vapor = 3 phases (vapor is not steam)
Mixed gasses = just 1 phase
a saturated salt solution has 3 phases: solution, undissolved salt, vapor
example of phase change: water becoming vapor |
|
|
|
a homogenous substance is? |
uniform throughout it's volume. propeties are the same throughout sodium chloride solution is homogenous soup is homogenous (apart from when it's cold around the edges and warm in the middle) does not imply same molecule |
|
|
|
how do you increase the rate of vaporization (liquid to gas?) |
increase temperature reducing pressure both average KE is constant. but some atoms are moving fast enough to break away from others and pass into vapor state in a closed vessel, the vapor can't escape, and most reverts back to liquid. the amount of molecules leaving at a given time equal the amount that return. = equilibrium the vaporised liquid forms a partial pressure in the closed vessel (contribute to the total pressure) |
|
|
|
vapor pressure is |
pressure of gas molecules (e.g. air) / liquid formed by evaporation at equilibrium (of gas phase condensing back into liquid phase) |
|
|
|
What creates a volatile substance? |
weak intermolecular forces = volatile substance strong intermolecular forces (e.g. h-bonds (which are nrot van-der-waals's forces, btw) = nonvolatile substance |
|
|
|
what is the difference between boiling and evaporation? |
boiling: vapor escapes with suficcient pressure to push back any other gas present, rather than diffusing through it so acts more like a bullet than a pinball vapor pressure increases with temperature. at boiling point, vapor pressure is equal to opposing external pressure |
|
|
|
if you boil something in a closed vessel, what will happen? |
vapor pressure will exceed air pressure, and boiling will occur. vaporization will occur. the pressure will soon build up within that vessel. boiling will slow if the gas pressure exceeds the vapor pressure |
|
|
|
what effect does molecular weight have on boiling point?? |
boiling point increases. it takes more energy to push the molecule into the atmosphere seen with alkanes |
|
|
|
freezing point is? |
when the vapor pressure of the solid is equal to that of the liquid pure, crystaline solids melt completely at 1 temperature impure solids begin to melt at one temperature, but become completely liquid at a higher temperature |
|
|
|
at melting/boiling point, what does extra heat energy do? |
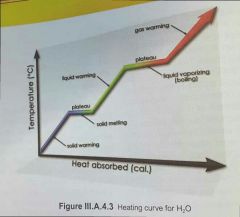
it does NOT raise the temperature. temperature is uniformly raised with heat up unil melting/boiling point. then it stops, until the melting/boiling process in increased the heat energy during these plateus is used to break intermolecular bonds and convert ice to water / water to vapor. there is no change in temperature during a phase change there is an increase in the PE (potential energy) of molecules instead during phase changes [as opposed to an increase i avarege kinetic energy] |
|
|
|
what is the amount of energy to change 1 mole of a substance from solid to liquid / liquid to gas called? |
the molar heat of fusion the molar heat of vaporization each phase has it's own amont of heat required. |
|
|
|
Which has more enthalpy [does he mean energy?], molar heat of fusion, or molar heat of vaporisation? |
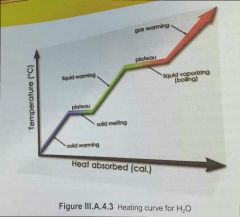
molar heat of fusion (solid to liquid) has greater enthalpy [energy?] because: more energy is required to break intermolecular bonds (liquid to gas) than to weaken intermolecular bonds (solid to liquid) |
|
|
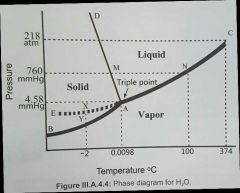
Describe this diagram
what are the boundries and what do they represent? |
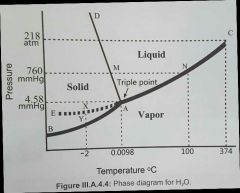
The boundries (curves) represent where two phases are in equilibrium. either side of the boundry, the substance exists in only 1 phase
Boundries: AB = sublimation / deposition AC = evaporation/condensation AD = melting/freezing
triple point = all 3 phases co-exist in equilibrium
C=critical point, where vapor and liquid are indistinguishable. M = true melting point for ice at 760mm/hg at triple point, vapor pressure of ice and water is the same
AE = metastable (beyond-stable) equilibrium between supercooled water and it's vapor: if T is slightly raised at point X, a little liquid will vaporise until a new equilibrium is established at that higher temperature
AB (sublimation/deposition) is more stable than AE as AB is at a lower energy
for clarity, the dotted lines are just extrapolations demonstrating significant points, such as at T=100 and P=760mmhg, vapor and liquidd water exist in equilibrium (= boiling point)
|

Triple point!! |
|
|
Critical point is? |

A supercritical fluid is any substance at a temperature and pressure above its critical point, where distinct liquid and gas phases do not exist. It can effuse through solids like a gas, and dissolve materials like a liquid. |
|
|
|
What is normal boiling point? |
the temperature at which a substance boils when the pressure is 1atm 100 degrees c for water (373 degrees k) |
|
|
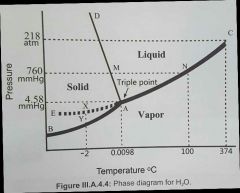
Slope AD shows? |
an increase in pressure creates a decrease in melting point
UNIQUE to water!! AD is usually a positive slope
an isothermal (same temperature) increase in pressure will compress ice into water!
uniquely, H2O is more dense in liquid form than solid form
H bonds and their cohesive nature mainly account for the high density of water. most substances increase their melting point with pressure (more densley packed, stronger intermolecular forces) - AD slants to the right for these. |
|

