![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
The nucleus of an atom is made up of what 2 things? |
Protons and neutrons |
|
|
|
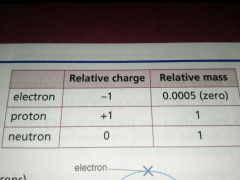
Electrons protons and neutrons have what relatively charge and relatively mass? |
|
|
|
|
What are isotopes? |
Elements with the same atomic number but different mass numbers are isotopes |
|
|
|
Remember |
Periodic table; charges go as follows by the columns +1 +2 +3 0 -3 -2 -1 0 |
|
|
|
Atoms with an outer shell of eight electrons have a what? |
Stable electronic structure |
|
|
|
How can atoms be made to have a stable electronic structure? |
Atoms can be made stable by transferring electrons. This is called ionic bonding. |
|
|
|
Atoms can have a stable electronic structure by transferring electrons. Whats this called? |
Ionic bonding |
|
|
|
Metal atoms always lose or gain electron to get a stable electronic structure? And is it positive or negative ion after that? |
Metal atoms always loses an electron to get a stable electronic structure and becomes positive ion |
|
|
|
Non metal atoms always lose or gain atom to get stable electric structure? And after ionic bonding, does it become negative or positive ion? |
Non metal atoms always gain an electron in ionic bonding to get stable electric structure. They become negative ions |
|
|
|
If an atom gains electron, a _____ ion forms. |
Negative |
|
|
|
If an atom loses an electron, a ______ ion forms. |
Positive |
|
|
|
Tip |
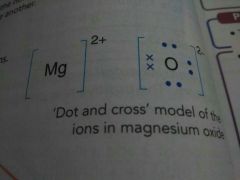
|
|
|
|
Positive ions and negative ions are held together by what? |
Attraction |
|
|
|
Non metals can share electron pairs between atoms. This is known as what? |
Covalent bonding |
|
|
|
periodic table; Newlands put 56 elements into groups and saw what? |
That every eighth element behaved similarly. This was not accepted for 50 years until other scientists discovered more evidence |
|
|
|
What did mendeleev do for periodic table? |
Arranged the elements in order in a table..he saw there were gaps in his pattern and predicted new elements would be found so he put gaps |
|
|
|
What are elements in group 1 properties? |
They act vigorously with water Hydrogen gas is given off Metal reacts with water to form an alkali - the hydroxide of the metal |
|
|
|
As you go down the group 1 in periodic table, the elements react with water more....? |
More vigorously |
|
|
|
Atoms of group 1 alkali metals have similar properties. Why? |
They all have one electron in their outer shell |
|
|
|
The easier it is for an atom of an alkali to lose one electron, the more it is what? |
More reactive it is |
|
|
|
Alkenes are what? |
Alkene is made of carbon and hydrogen. Hydrogen is always double amount of carbon.
E.g. C2H4 |
|
|
|
Unsaturated means? |
Has at least one double bond between carbons |
|
|
|
Whats alkali? |
Is a compound that dissolves to give a solution with pH higher than 7 |
|
|
|
If electrons are lost, what is the process called? |
Oxidation |
OILRIG |
|
|
If an atom becomes a positive ion, did oxidation or reduction take place? |
Oxidation |
|
|
|
Using flame test, how do you know if there's lithium in the compound? |
The colour of the flame is red |
What colour of the flame is it? |
|
|
When doing flame test, how do you know if sodium is present in the compound? |
Sodium = colour of flame is yellow |
|
|
|
If doing flame test, how do you if potassium is present in a compound? |
Potassium = colour of flame is lilac |
|
|
|
Why do group 7 elements have similar properties? |
Because they all got 7 electrons on outer shell |
|
|
|
The further the outer shell is to the nucleus, the easier it is for an atom to lose ____ _____. The easier it is to lose the _____, the more reactive the halogen is. (Group 7) |
Further the outer shell is to the nucleus, the easier it is for an atom to lose one electron . The easier it is to lose the electron, the more reactive the halogen is. |
|
|
|
How are group 7 elements called? |
Halogens |
|
|
|
Which one is the most reactive element in group 7? |
The ones at top (the reactivity increases as you go up the column in group 7) |
|
|
|
If the electrons are gained, what process is that? |
Reduction |
OIL RIG |
|
|
If reduction occur, the atom becomes a positive or negative ion? |
Negative ion |
|
|
|
In Haber process, what is used as catalyst and in what temperature? |
Iron 450°C |
|
|
|
Haber process makes ____ which is used in fertilisers. |
Haber process makes ammonia which is used in fertilisers |
|
|
|
Nick is used for what in the manufacture of margarine? |
Harden the oils |
|
|
|
If a [metal] carbonate is heated it undergoes thermal decomposition to form what? |
A [metal] oxide and carbon dioxide |
|
|
|
A property can be either physical or chemical. Give example for both. |
Physical - high thermal conductivity of copper Chemical - resistance to attacks by oxygen or acids shown by gold |
|
|
|
Give physical properties other than the high thermal conductivity of copper. (4) |
- lustrous -.shiny -.malleable - ductile |
|
|
|
Why do metals have high boiling point and melting point? |
Due to their strong metallic bonds |
|
|
|
Metallic bonds between atoms in metals are hard to break and what do they require to break? |
A lot of energy |
|
|
|
When metals conduct electricity, what do electrons do? |
Electrons in the metal move |
|
|
|
3 metals that are good conductors? |
Silver, copper and gold |
|
|
|
Remember |
A metabolic bond is a strong ng electrostatic force of attraction between close packed positive metal ions and a 'sea' of delocalised electrons |
|
|
|
Why do metals conduct electricity ? |
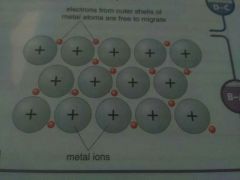
A metal conducts electricity because delocalised electrons within its structure can move easily |
|
|
|
What are superconductors? |
Superconductors are materials that conduct electricity with little or no resistance |
|
|
|
Example of superconductor? |
Mercury |
|
|
|
The electrical resistance of mercury suddenly drops to zero at -268.8°C. This phenomenon is called what? |
Superconductivity |
|
|
|
When a substance goes from it's normal state to a super conducting state, what does it no longer have inside it? |
It no longer has any magnetic fields inside it |
|
|
|
If a small magnet is brought near the superconductor, what happens ? |
It will be repelled |
|
|
|
What is repelled ? |
Force pushing you back. E.g. when you put + + magnetics to each other, they repel. |
|
|
|
If a small permanent magnet is placed above the superconductor, what happens? |
It levitates |
|
|
|
3 potential benefits of superconductors are what? |
- loss-free power transmission - super-fast electronic circuits - powerful electromagnets |
|
|
|
Disadvantages of superconductors ? |
They work only at very low temperatures; limits their use Superconductors that function at 20°C need to be developed |
|
|
|
To turn water into clean water, what process does it need to go through? |
Water purification |
|
|
|
After water purification treatment, the water can be polluted by what pollutants? (2) |
* fertilisers such as nitrates and pesticides from crop spraying get into the water before water purification and then the water needs go through other treatment to get rid of them * old water pipes made of lead can affect the water |
|
|
|
What are the three main stages in water purification? |
• sedimentation - chemicals are added to make solid particles and bacteria settle out • filtration of very fine particles - a layer of sand on gravel filters out the remaining fine particles; some types of sand filter also remove microbes • chlorination - chlorine is added to kill microbes |
|
|
|
Disadvantage of water purification? |
It takes energy to pump and purify which increases global warming |
|
|
|
Why we don't take sea water to turn into clean water? |
It's expensive as it not only needs to go through water purification but also distillation in order to remove dissolved substances and this takes a huge amount of energy . Only used when there's no water |
|
|
|
Whats the further water treatment after water purification if there's dissolved substances present? |
Distillation |
|

