![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
34 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Define first ionisation energy |
The first ionisation energy is the energy needed to remove 1 mole of electrons from 1 mole of gaseous atoms. |
|
|
|
Which factors affect ionisation energy? |
• Nuclear charge - The more protons there are in the nucleus, the more positively charged the nucleus is. • Atomic radius - An electron further away is less atteracted to the nucleus. • Shielding - Outer electrons feel less attracted when there more shells between them and the nucleus. |
|
|
|
Show the second ionisation energy of oxygen. |
O^+(g) -> O^2+(g) + e^- |
|
|
|
How would you draw the first ionisation of Sodium? |
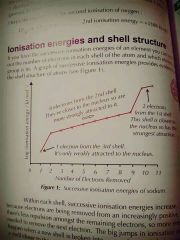
|
|
|
|
Name examples of giant covalent lattices (macromolecular stuctures). |
Carbon and Silicon |
|
|
|
What are the three carbon allotropes? |
Graphite, diamond and graphene. |
|
|
|
How are the carbon atoms in grahite held together? |
They are covalently bonded with three bonds each. The forth outer electron of each carbon atom is delocalised, which allows them to be held by induced dipole-dipole interactions. |
|
|
|
What properties does graphite have due to it's structure? |
• The weak forces between layers of graphite are easily broken so the sheets can slide over each other. • The delocalised electrons in graphite are free to move, so am electric current can flow. • The layers are far apart compared to the covalent bonds, so graphite has a low density. • Because of the covalent bonds they have a very high melting point. • Graphite is insoluble in any solvent. |
|
|
|
Define sublime |
It changes straight from a solid to a gas, skipping the liquid stage. |
|
|
|
What is diamond made up of? |
They are made up of carbon atoms that are covalently bonded to 4 other carbon atoms in a tetrahedral shape. |
|
|
|
Why does having strong covalent bonds help the structure of diamond? |
• High melting point - sublime over 3800K. • Hard - used in saws etc. • Good thermal conductor - vibrations travel easily through a stiff lattice. • Can't conduct electricity - all outer electrons are involved in bonds. • Diamond doesn't dissolve. |
|
|
|
What is graphene? Give a property and how it can be used. |
One layer of graphite - it's a sheet of carbon atoms. It is transparent it can be used as touchscreens on phones etc. |
|
|
|
What are giant metallic lattice structures made up of? |
They are a lattice of metal ions surrounded by a 'sea' of delocalised electrons. |
|
|
|
How are the following affected by metallic structures: • Melting and Boiling points • Ability to be shaped • Conductivity • Solubility |
• Melting and boiling points - the number of delocalised electrons per atom affects the melting and Boiling points. The more they are, the higher the the melting and boiling points are. • Ability to be shaped - metal ions can slide over each other when the structure is pulled. • Conductivity - Delocalised electrons can pass on kinetic energy, which makes theme good thermal conductors. • Solubility - Metals are insoluble because of the strength of the metallic bonds. |
|
|
|
In group 2, what happens to reactivity down the group? |
It increases because it's easier to lose electrons, so it is more reactive. |
|
|
|
How do group 2 elements react, in terms of oxidation? |
M -> M^2+ 2e^- |
|
|
|
How group 2 elements react with the following: • Water • Oxygen • Dilute acids |
• Water - they form to make a metal hydroxide and hydrogen. The metal hydroxide can dissolve in water to form hydroxide ions. • Oxygen - they form to make solid white oxides. These oxides would dissolve in water to for hydroxide ions. • Dilute acids - they form metal chloride/sulfate and hydrogen. |
|
|
|
Name 2 uses of group 2 compounds. |
• Calcium hydroxide is used in agriculture to neutralise acid soils. • Magnesium hydroxide and calcium carbonate are used in some indigestion tablets as they neutralise excess stomach acids. |
|
|
|
Give the formula, colour, physical state and electronic structure of the halogens. |

|
|
|
|
Explain the trend of boiling points in the halogens. As well as volatility. |
It increases as you go down the group, as there is increasing Trentham due to the induced dipole-dipole forces as the no. of electrons increase. Volatility is high when there's a low boiling point, so volatility decreases down the group. |
|
|
|
How do halogens react? |
By gaining an electron in their outer shell, this means they are reduced. So halide ions are formed. They're oxidising agents. X + e^- -> X^- |
|
|
|
What happens when a halogen is dissolved in an organic solvent? |
A colour is formed: • A violet/pink shows the presence of iodine. • An oranage/red colours shows bromine. • A very pale yellow/green shows chlorine. |
|
|
|
How could you test for a halogen? |

|
|
|
|
What should happen to the halogens when they are mixed with ammonia? |

|
|
|
|
Define disproptionation |

When a a single element is simultaneously oxidised and reduced. The halogens undergo disproptionation when they react with cold dilute alkali solutions. |
|
|
|
How can you make bleach? |
When you mix a metal hydroxide with chlorine it forms to make a chlorate ion, which is known as bleach. |
|
|
|
How does chlorine and water undergo disproptionation? |
Chlorine mixes with water and forms hydrochloric acid and chloric acid. |
|
|
|
How is chlorine involved in water treatment? |

|
|
|
|
What other alternatives are there to chlorine? In terms of water. |
• Ozone (O3) is a strong oxidising agent that is great at killing microorganisms. It is expensive to produce and isn't permanent. • Ultraviolet light kills microorganisms by damaging their DNA. Same weaknesses as Ozone. |
|
|
|
How would you test for carbonate ions? |

|
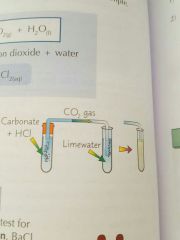
|
|
|
Which order should an unknown substance be tested? |

|
|
|
|
How would you test for sulfates? |

|
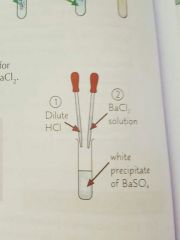
|
|
|
How would you test for Ammonium ions? |

|

|
|
|
How would you test for halides? |

|
|

