![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is a chemical bond? |
A mutual attraction between the electron and protons of different atoms |
|
|
|
Potential energy vs internuclear distance between two atoms diagram |

|
|
|
|
Non polar covalent |

"Perfect sharing" electrons stay mostly in middle of bond |
|
|
|
Electronegativiy |

Ability to pull on a shared pair of electrons |
|
|
|
Electronegativiy trend |
Left - right increase Top - bottom decrease |
|
|
|
Partial charge |
Apparent gain or loss of charge |
|
|
|
Polar covalent bond |

Bonding electrons shared unequally between two atoms. Partial charges on atoms. |
|
|
|
Ionic bond |

Complete transfer of one or more valence electrons. Full charges on resulting ions |
|
|
|
Incomplete octects |

|
|
|
|
Lewis dot structures |

|
Definition and what do dots represent |
|
|
Expanded octets |

|
|
|
|
Endo/ Exo thermic |
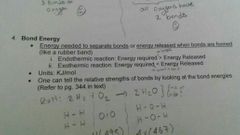
|
|

