![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
What is an Ionic Bond?
|
When an electron is transferred from a metal to a non-metal, an ionic bond is formed
|
|
|
Metals have ______ ionization energies (IE) and they prefer to ______ e-'s and become ______
|
low
lose cations |
|
|
Non-metals have ______ ionization energies (IE) and they prefer to ______ e-'s and become ______
|
high
gain anions |
|
|
What happens to the atomic radii when a cation is formed? Why?
|
Z_eff increases due to decreased shielding, & remaining e-'s are held tighter
|
|
|
What happens to the atomic radii when a anion is formed? Why?
|
Z_eff decreases due to increased shielding, & each e- is held a little looser
|
|
|
What happens when a non-metal gains e-'s? What happens to the name?
|
Becomes an anion
Change name by adding "-ide" to the end chlorine --> chloride oxygen --> oxide sulfur --> sulfide |
|
|
Non-metals want to ______ e-'s in order to ______
|
gain, fill their outer shell
|
|
|
When a metal ______ e-'s, it becomes a ______. What happens to the name?
|
loses, cation
add the word "ion" after the atom name sodium --> sodium ion calcium --> calcium ion |
|
|
The more EN atom is always the ______
|
anion
|
|
|
Describe some of the general trends of the periodic table
|
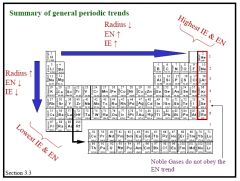
|
|
|
What is the oxidation state
|
the charge on an atom assuming the bonds are ionic
|
|
|
Rules of oxidation states
|
-total charges from each atom must add up to the overall charge on the substance
-the most electronegative atom will be the anion |
|
|
Steps for determining the oxidation states
|
1. Choose the most electronegative atom. This is anion.
2. Assign oxidation states to unambiguous atoms. 3. All other oxidation states must be calculated so that the total charge equals to the overall charge |
|
|
What are polyatomic ions?
|
charged groups of covalently bound atoms
usually made up of nonmetals many are oxoanions |
|
|
What are oxoanions?
|
An atom bound to one or more oxygens
|
|
|
When does an oxoanion have the suffix "-ate"
|
When the non-oxygen atom is in the highest possible oxidation state
**Remember "ate" = "at" Group #** NO_3 - = nitrate (N ox.st. = 5+) |
|
|
When does an oxoanion have the suffix "-ite"
|
When the non-oxygen atom is in the next lower oxidation state (Group # -2)
**Remember the i in "ite" has two marks in letter, thus the ox.st. = Group# - 2** NO_2 - = nitrite (N ox.st. = 3+) |
|
|
When an electron is transferred from a metal to a non-metal, an________is formed
|
ionic bond
|

