![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
- 3rd side (hint)
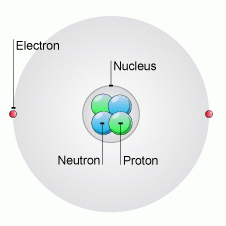
List 3 facts |
1. Every atom is made of a nucleus consisting of protons and neutrons.
2.The nucleus is surrounded by electrons. 3.Protons and electrons are oppositely charged. Neutrons have no charge |
Remember charges and positions (that what she said)! |
|
|
Comparing the charge of electrons, neutrons and protons. |
Proton charge has a 1+ charge. Neutron has a neutral charge. Electrons have a -1 charge. |

|
|
|
Isotopes |
The full chemical symbol for an element shows its mass number at the top and atomic number at the bottom. Here is the full symbol for an isotope of carbon, carbon-12 or 12C.
|
It tells us that a carbon atom has:Six protons (because its proton number, at the bottom, is 6)Six electrons (because the number of protons and electrons in an atom is the same) |

