![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
21 Cards in this Set
- Front
- Back
|
reaction where products have less energy than reactants.... |
they must have transferred excess energy usually by heating |
|
|
exothermic reactions |
energy ➡ surroundings rise in temp of surr. eg. combustion, neutralisation, oxidation. uses - hand warmers oxidation of iron in air with salt solution catalyst - self heating cans |
|
|
exothermic energy profile |
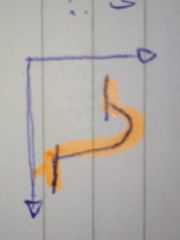
|
|
|
endothermic reactions |
takes in energy from surr. fall in temp of surr. much less common eg. thermal decomposition citric acid + hydrogencarbonate uses - ice pack more convenient than ice, more flexible |
|
|
endothermic energy profile |
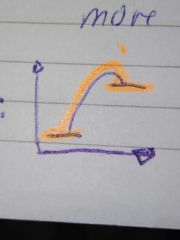
|
|
|
REQUIRED PRACTICAL energy transfer of reactions |
lid - stops evaporation polystyrene cup - insulator cotton wool - insulation measure temp change diff variables see whether endo or exo solid + liquid or 2 liquids |
|
|
reaction energy profiles |
▪️overall energy change - diff in height between reactants n products ▪️whether endo or exo ▪️activation energy (Ea) - energy needed to break binds n start reaction. min amnt of energy reactants need to react |
|
|
units of energy and energy transfer |
1000J = 1kJ transfer = kJ/mol (mol of reactants) |
|
|
bond breaking reaction = |
endothermic |
|
|
bond making - |
exothermic |
|
|
bond energy calculations |
bond energies will be given 1. write out all displayed formulas to see all bonds 2. Work out energy used in bond breaking - bond energies of all reactants 3. energy given out in bond making - bond energies of all products 4. use formula for energy change |
|
|
energy change formula |
e.c. = breaking - making energy energy overall is positive = exo ^ negative = endo |
|
|
cells & batteries |
▪️2 electrodes - conduct (Cu+ Zn) ▪️electrolyte - liquid that conducts n contains ions that react w electrodes ▪️reactions set up charge diff. ▪️electrodes connected by wire ▪️charge can flow between ▪️voltmeter |
|
|
electrodes n voltage |
charge difference bigger diff in reactivity of electrodes = bigger voltage use data to make reactivity series if left electrode is less reactive, voltage is positive |
|
|
electrolytes and voltage |
lower conc. of ions in electrolyte = lower voltage as ions react conc. decreases (so will voltage) until zero and cell stops working |
|
|
batteries |
2 or more cells in series voltage of battery = sum of voltages of all cells |
|
|
(non) rechargeable batteries |
irreversible reactions ➡ reacting particles used up ➡ reaction can't happen in a rechargeable cell, the reaction can be reversed |
|
|
fuel cells |
fuel enters cell is oxidised set up p.d. powers device oxygen enters and reacts to form water |
|
|
hydrogen - oxygen fuel cell |
electrolyte often potassium hydroxide electrodes often carbon w catalyst anode - hydrogen - loses electrons ➡ H+ ions ➡ oxidation ➡ ions move to cathode cathode - oxygen - gains electrons ➡ reacts w H+ ions ➡ water ➡ reduced anode ➡ cathode current redox: 2H2 + O2 ➡ 2H2O half: +: H2 ➡ 2H2 + 2e- - : O2 + 4H+ + 4e- ➡ 2H2O |
|
|
hydrogen fuel cells vs batteries ✔️ |
✔️less pollutants - only by products are water n heat - batteries are polluting to dispose of made of toxic metals ✔️no limit to how many times rechargeable ✔️store more energy than batteries so recharged less often
|
|
|
hydrogen fuel cells vs batteries ❌ |
❌ hydrogen is gas - takes up more space ❌ hydrogen explosive when mixed w air - hard to store ❌ H-fuel either made from hydrocarbons (fossil fuel) or by electrolysis (electricity made by f.f.) |

