![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
8 Cards in this Set
- Front
- Back
|
Formic acid |
Methanoic acid (1 C) |
|
|
Acetic acid |
Ethanoic acid (2 carbons) |
|
|
Z E |
Zame zide Eway |
|
|
Dicarboxylic acids No space 1space 2 space |
Oxalic acid Malonic acid Succinic acid |
|
|
Carboxylic acids MP and BP? Solubility? More acidic than? Due to? |
-High due to strong intramolecular forces -Inwater just like alcohol -Alcohol and water. This is due to inductive effects of carbonyl and res stabilization of carboxylate ion |
|
|
Miscelles Head Tail |
Head polar (hydrophilic waterliking) Tail nonpolar (phobic watrr hatel |
|
|
Simpliest dicarboxylic acid |
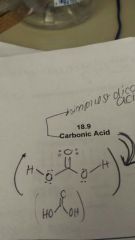
Carbonic acid |
|
|
Lactones |
Cyclic esters |

