![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
80 Cards in this Set
- Front
- Back
|
Law v. Theory
|
Law summarizes a series of related observations. Theory gives underlying reasons for them. |
|
|
Law of Definite Proportions
|
Regardless of the amount of a compound, the ratio of components remain the same.
|
|
|
Law of Multiple Proportions
|
For different molecules
|
|
|
Dalton's Atomic Theory
|
1) each element is composed of indestructible atoms 2) All atoms of an element have the same mass and other properties that distinguish them from other elements 3) Atoms combine in whole number ratios to form compounds 4) Matter is neither created nor destroyed only rearranged |
|
|
Atomic Mass Calculation
|
Atomic Mass = Sum(fraction of isotope x mass of isotope)
|
|
|
Accuracy v. Precision
|
Accuracy: closeness to true value Precision: closeness of a set of values to each other |
|
|
Intensive Properties
|
Characteristics of substances that do not change with the amount present.
|
|
|
Density
|
Density = mass/volume
|
|
|
Law of Conservation of Energy
|
Energy cannot be created or destroyed only converted
|
|
|
Electromagnetic Radiation, speed of light
|
c=lamda * nu c: 3.00x10^8 lamda: wavelength nu: frequency |
|
|
Energy of a Photon
|
E = h * nu or E = h*c/lamda h=6.626x10^-34Js |
|
|
Energy Related to Wavelength Energy Related to frequency |
Increase wavelength -> decrease energy Decrease wavelength -> increase energy Increase frequency -> increase energy Decrease frequency -> decrease energy Increase wavelength = decrease in frequency |
|
|
Destructive v. Constructive Interference
|
Destructive: waves out of phase, cancel each other Constructive: waves in phase, combine |
|
|
Electromagnetic Radiation Energy
|
Red, least energy, lrg wavelength Orange Yellow Green Blue Indigo Violet, most energy, sml wavelength |
|
|
de Broglie Equation (wave behavior of photons)
|
lambda = h/(mv) h=6.626x10^-34Js m=mass in kg v=velocity m/s |
|
|
Heisenberg's Uncertainty Principle
|
The accurately you know the position of a small particle, e-, the less you know about its speed, and vice versa
|
|
|
Schrodinger's Equation
|
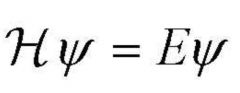
Calculates probability of finding an electron
|
|
|
Quantum Numbers
|
n: Principal level (shells) l: Sublevels = 0...n-1 (0=s, 1=p, 2=d, 3=f) ml: Orbitals = -l,...,0,...,+l ms: Spin = +1/2 or -1/2 |
|
|
Sublevel l=0
|
S orbital Lowest nrg orbital in principle nrg state Shape: sphere Nodes: n-1 |
|
|
Sublevel l=1
|
porbital > n=1, three p orbitals Shape: dumbbell on x, y, and z axis Nodes: n=1; 1, n=2; 2, etc. |
|
|
Sublevel l=2
|
d orbitals > n=2, five d orbitals Shape: 4 dumbells on each plane, 1 on xy axises and 1 w/ dumbbell/donut Nodes: 2 nodal planes, 1 addl each higher level |
|
|
Sublevel l=3
|
f orbitals 7 orbitals |
|
|
Pauli Exclusion Principle
|
Each orbital can hold only 2 e- and must have opposite spins.
|
|
|
Coulomb's Law
|
Increase nrg, increase charge/decrease radius Decrease nrg, decrease charge/increase radius E proportional to Charge/radius |
|
|
Hund's Rule
|
Orbitals with same energy are filled w/ one each before completing pairs
|
|
|
Paramagnetic v. Diamagnetic
|
Paramagnetic: contains unpaired electrons Diamagnetic: electrons are paired |
|
|
Periodic Properties
|
1A: Alkaline metals 2A: Alkaline Earth metals 7A: Halogens 8A: Noble Gases Elements in same column share common properties |
|
|
Periodic Table Trends, Radius
|
Down column atomic radius increases Right in row atomic radius decreases. Transition metals relatively the same size cations << atom and anions >> atom |
|
|
Effective Nuclear Charge
|
Zeff = Z - S Z: Actual nuclear charge (# protons) S: Charge screened by other e- (non valence e-) |
|
|
Periodic Trends, Ionic Energy
|
Energy required to remove an e- For IE1: - down column, IE1 decreases - to right of row, IE1 increases |
|
|
Periodic Trends, Electron Affinity
|
How easily an atom accepts an addl e- Down column, no trend Right of row, more negative |
|
|
Periodic Trends, Metallic Character
|
Down column, metallic character increases Right of row, metallic character decreases |
|
|
Ionic Bonds
|
Bond between Metal and Nonmetal Txfr of e- |
|
|
Covalent Bond
|
Bond between 2 or more nonmetals Sharing of e- |
|
|
Lattice Energy
|
LE is proportional to charge/radius increase charge, increase LE increase radius, decrease LE Look at charge 1st |
|
|
Electronegativity
|
Ability of an atom to attract electrons to itself in a chemical bond. Down column, EN decreases Right of row, EN increases FONClBrISCH |
|
|
Bond Polarity
|
Non polar covalent: EN < 0.4 Polar covalent: EN = 0.4 - 2.0 Ionic: EN > 2.0 |
|
|
Lewis Dot Resonance, Formal Charge
|
Formal Charge = (No. valence e-) - (nonbond e- + 1/2 bonding e-)
|
|
|
sp3 Hybridization
|

Tetrahedral shape
|
|
|
sp2 Hybridization
|

Trigonal planar
|
|
|
sp Hybridization
|
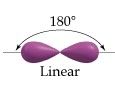
Linear
|
|
|
sp3d Hybridization
|

Trigonal Bipyramidal
|
|
|
sp3d2 Hybridization
|
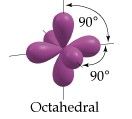
Octahedral
|
|
|
Molecular Bonding Theory
|
Electron orbits represent whole molecule
|
|
|
Bond Order
|
BO= (#bonding MOs - #antibonding MOs)/2
|
|
|
Molecular Orbitals for B2, C2, N2
|
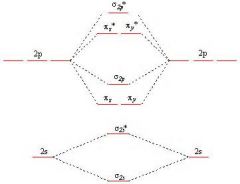
|
|
|
Molecular Orbitals for O2, F2, Ne2
|
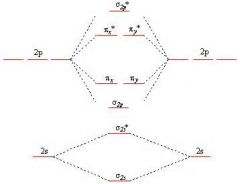
|
|
|
Strong Electrolytes
|
Ionic compounds that completely dissociate into ions in water
|
|
|
Strong Acids
|
Acids that completely ionize in solution. HCl HBr HI HNO3 H2SO4 HClO4 |
|
|
Weak Acids
|
Acids that don't completely ionize in solution HC2H3O2 HF |
|
|
Strong Base
|
Base that completely ionizes in solution NaOH LiOH KOH Ca(OH)2 Ba(OH)2 |
|
|
Weak Base
|
Base that doesn't completely ionize in solution NH3 |
|
|
Solubility Rules, Soluble
|
All Soluble Li+, Na+, K+ and NH4+ all soluble NO3- and C2H3O2- all soluble Cl-, Br- and I- not w/ Ag+, Hg2 2+ or Pb2+ SO4 2- not w/ Ca2+, Sr2+, Ba2+, Pb2+, Hg2 2+ |
|
|
Solubility Rules, Insoluble
|
All Insoluble OH- and S2- not w/ Li, Na, K, NH4 S2- not w/ Ca2+, Sr2+, Ba2+ OH- not w/ Ca2+, Sr2+, Ba2+ CO3 2- and PO4 3- not w/ Li+, Na+, K+ and NH4+ |
|
|
Aqueous Equations
|
Molecular: complete eqn Complete Ionic: all species in soln Net Ionic: species that change during rxn |
|
|
Naming oxyacids
|
-ate -> ic -ite -> ous |
|
|
Redox Reactions, Assigning Oxidation States
|
1) free element = 0 2) ion = charge (Cl- = -1, Sr2+ = +2) 3) sum ox states of all atoms (in neutral molecule = 0, in ion is charge of ion) 4) 1A metals = +1, 2A = +2 5) F =-1, H=+1, O=-2, 7A=-1, 6A=-2, 5A=-3 |
|
|
State Function
|
Value only depends on state of the system. Dependent on initial values and final values. |
|
|
Energy
|
Delta E = q + w
|
|
|
Exothermic Endothermic |
Release of energy by the system (-q & -w) Absorption of energy by the system (+q & +w) |
|
|
Enthalpy of Rxn
|
Delta Hrxn = Sum(bonds broken) + Sum(bonds formed)
|
|
|
Standard Heat of Formation
|
Delta Hf = Sum(products) - Sum(reactants) Pure compound: enthalpy change when 1 mole of compound forms from its constituent elements. Pure element: =0 |
|
|
Kinetic Molecular Theory
|
1) size of particle is negligibly small 2) KEave is proportional to the temperature in K 3) All collisions are elastic |
|
|
Nature of Pressure
|
P=F/A Particles collide with a surface exerting a force on the surface. This force over an area is = Pressure |
|
|
Boyle's Law
|
P1V1=P2V2 A decrease in the volume of gas causes the gas particles to occupy a smaller space, increasing the number of collisions with surrounding surface thereby increasing pressure |
|
|
Charles' Law
|
V1/T1 = V2/T2 An increase in temperature causes an increase in KE which means an increase in speed resulting in an increase in pressure. To maintain pressure, volume must increase. |
|
|
Avogadro's Law
|
V1/n1 = V2/n2 Increasing the number of particles results in the increase in collisions. To maintain same amount of collisions (pressure) volume must increase. |
|
|
Dalton's Law
|
Ptot=P1 + P2 +...Pn Particles are negligible in size and do not interact. Adding different particles has same effect as adding more particles |
|
|
Graham's Law of Effusion
|
rateA/rateB = (MB/MA)^1/2
|
|
|
Van der Waals Equation
|
Ideal gas law does not apply at high pressures (vol) and low temperatures (press) [P+a(n/V)^2]x[V-nb]=nRT intermolecular forces: P+a(n/V)^2 volume: V-nb |
|
|
Intermolecular Forces
|
Dispersion Forces: weakest Dipole-Dipole Forces H-Bonding Forces Ion-Dipole Forces: strongest Increased intermolecular forces = higher MP and BP |
|
|
Dispersion Force (LDF)
|
Temporary dipole Strength is dependant upon mass and surface area |
|
|
Dipole-Dipole Forces
|
Between polar molecules
|
|
|
H Bonding Forces
|
Super dipole-dipole force. Occurs w/ H and F, O or N atoms |
|
|
Ion-Dipole Force
|
Ionic compound w/ polar compound
|
|
|
Volatility
|
Liquids ability to vaporize Increase temp -> increase rate of vaporization Increase surface area -> increase rate of vap Decrease intermolec forces -> increase rate of vap |
|
|
Liquid to Gas Gas to Liquid |
Liquid to Gas: (vaporization) - Delta Hvap (kJ/mol) Gas to Liquid: (condensation) - -Delta Hvap (kJ/mol) |
|
|
Solid to Liquid Liquid to Solid |
Solid to Liquid: (fusion/melting) - Delta Hfus (kJ/mol) Liquid to Solid: (freezing) - -Delta Hfus (kJ/mol) |
|
|
Critical Point
|
Point at which gas cannot condense to a liquid, no matter the pressure. Properties of both gas and liquid states. |
|
|
Sublimation Deposition |
Solid to Gas Gas to Solid |

