![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
23 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
For the following reaction, Kc is 144 at 200°C. If .4 mol of both A and B are placed in a 2 liter contained at that temperature, what will be the concentration of B at equilibrium? |
.015 M |
|
|
|
When the above system is at equilibrium at 200°C, the concentrations are found to be: [A] = .2 M, [B]= .3 M, [C]= .3 M If the volume of the container is suddenly doubled at 200°C, what will be the new concentration of A? |
.07 M |
|
|

Consider the following reaction. What would be the equilibrium constant expression? |
Kc = ([CBr4][HBr]^4)/([Br2]^4[CH4]) |
|
|
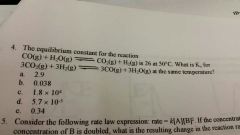
The equilibrium constant for the reaction... |
5.7 × 10^-5 |
|
|
|
Consider the following rate law expression: rate = k [A][B]^2 If the concentration of A is triple and the concentration of B is doubled, what is the resulting change in the reaction rate? |
The rate is increased by a factor of 12. |
|
|
|
At a certain temperature Kc= 25 and a reaction vessel contains a mixture with the following concentrations: [H2]= .1 M, [Br2]= .1M, and [HBr] = .5 M. Which of the following statements concerning the reaction and the reaction quotient, Q, is true? |
Q = Kc |
|
|
|
The decomposition of dinitrogen pentoxide obey the rate-law expression: rate = .08 min^-1 [N2O5]. If the initial concentration of N2O5 is .3 M, what is the concentration after 2.6 minutes? |
.24 M |
|
|

|
A. |
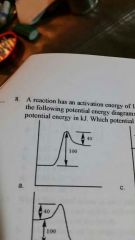
|
|
|
Consider the formation of ammonia from nitrogen and hydrogen. A reaction is initiated with .5 M of both N2 and H2 and no NH3. At equilibrium, the concentration of NH3 was .25 M. What is the value of Kc? |
85 |
|
|
|
The rate constant for the reaction 2NO2 -> N2O4 is 2.79 L/mol•min at 48°C. If the initial concentration of NO2 is 1.05 M, what is the half-life? |
20.5 s |
|
|
|
The gas phase reaction A+B+C -> D has a reaction rate which is experimentally observed to follow the relationship rate = k [A]^2[C]. The reaction is ____order in A, ___ order in B, and ___ order in C. |
Second, zero, first |
|
|
|
The specific rate constant, k, for a reaction is .44 s^-1 at 25°C, and the activation energy is 245 kJ/mol. Calculate k at 125°C |
2.71 × 10^10 s^-1 |
|
|
|
Which idea listed below is not a part of the collision theory of reaction rates? |
All molecular collisions result in a reaction. |
|
|
|
Which of the following statements concerning a reaction and it's mechanism is false? |
For a multi-step mechanism, the fastest step is the rate-determinimg step. |
|
|
|
The reaction 2CH4 -> C2H2 + 3H2 has a rate constant of 5.76 M^-1 •min^-1 at 1600 K. How long would it take for the concentration of CH4 to be reduced from .89 M to 5.25 × 10^-4 M? |
5.51 hours |
|
|
|
A catalyst |
Increases the rate at which equilibrium is reached without changing the equilibrium constant. |
|
|
|
Reaction rates increase with increasing temperature because ____. |
A greater fraction of molecules posses the activation energy when they collide. |
|
|
|
For the system H2 + CO2 <--> H2O + CO at equilibrium, the addition if H2 would cause ____. |
More H2O and CO to form. |
|
|
|
Of the following questions, which ones are kinetic rather than thermodynamic concepts? |
If a reaction occurs, how fast will it occur? And What is the mechanism by which the reaction occurs? |
|
|
|
Which of the following is the best definition of chemical equilibrium? |
A condition where the forward and reverse reaction rates of a reversible reaction are equal and constant. |
|
|

Given the following data for the NH4 + NO2 -> N2 + 2H2O reaction, the rate law for the reaction is ____. |
Rate = k[NH4+][NO2-]^2 |
|
|
|
Suppose we let the reaction below come to equilibrium. Then we decrease the total pressure, by increasing the volume of the container. What will be the effect on the net amount of SO3 present? |
It decreases. |
|
|
|
What is the value of Kc for the reaction CH4 + H2O <--> CO + 3H2 if at equilibrium [CH4] = .2 M, [H2O] = .2 M, [CO] = .5 M, and [H2] = 1.5 M? |
42 |
|

