![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
15 Cards in this Set
- Front
- Back
|
Equilibrium |
statereached when the concentrations of reactants and products remain constant |
|
|
Equilibrium Constant (Kc) |
a ratio of concentration of products/ concentration of the reactants.
If the K <1 reaction moves left if the K > 1 reaction moves right |
|
|
Equilibrium Equations |
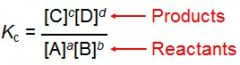
|
|
|
Kinetics and Equilibrium |
P is the partial pressure of a component |
|
|
Kp and its relationship to Kc |
/ |
|
|
Reaction Quotient: Direction of reaction |
? |
|
|
Finding Equilibrium Concentrations |
? |
|
|
Equal initial concentrations |
1.Finding Equilibrium Concentrations |
|
|
Unequal initial concentrations |
1.Finding Equilibrium Concentrations |
|
|
Partial Pressures |
1.Finding Equilibrium Concentrations |
|
|
Le Châtelier’s Principle |
? |
|
|
Concentration effects |
Le Châtelier’s Principle |
|
|
Volume effects |
Le Châtelier’s Principle |
|
|
Pressure effects |
Le Châtelier’s Principle |
|
|
Temperature effects |
Le Châtelier’s Principle |

