![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
16 Cards in this Set
- Front
- Back
|
Energetics of Solution Formation
|
ΔHsol’n = ΔHsolute [+] + ΔHsolvent [+] + ΔHmix [-]
--Also-- ΔHsol’n = ΔHsolute [+] + ΔHhydration [-] |
|
|
Henry's Law
|
Sgas = kHPgas
*Sgas is solubility of gas *kH is Henry's constant *Pgas is partial pressure of gas (usually in atm) |
|
|
Mass percent
|
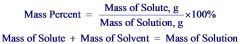
|
|
|
Percent Concentration
|

|
|
|
Volume Percent
|

|
|
|
Raoult's Law
|
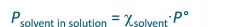
Psolution is vapor pressure of solution
P0 is vapor pressure of pure solvent |
|
|
raoult's law for volatile solute
|

|
|
|
Deviations from Raoult's Law
|

|
|
|
Freezing Point Depression
|

|
|
|
Boiling Point Elevation
|

|
|
|
graphic-freezing point depression and boiling point elevation
|
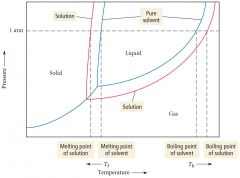
|
|
|
Osmosis
|
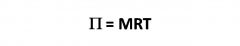
|
|
|
Van't Hoff Factor
|
i= (moles of particles in solution)/(moles of formula units dissolved)
|
|
|
Boiling Point Elevation- Ionic Solutions
|
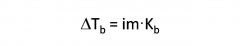
|
|
|
Freezing Point Depression- Ionic Solutions
|
|
|
|
Freezing Point Depression- Ionic Solutions
|
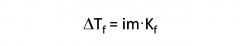
|

