![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
|
Solution = |
Solvent + Solute
- solution: homogeneous mixture (aqueous when water is the solvent)
- solvent: dissolving substance
- solute: dissolved substance |
|
|
Solubility |
- max amount of solute that dissolves in a given amount of solvent - varies with pressure and temperature |
|
|
Gaseous Solution Phases |
- solute phase: gas
- solvent phase: gas
- example: air (nitrogen and oxygen) |
|
|
Liquid Solution Phases |
1) solute phase: gas solvent phase: liquid example: soda (carbon dioxide in water) 2) solute phase: liquid solvent phase: liquid example: vodka (ethanol in water) 3) solute phase: solid solvent phase: liquid example: seawater (potassium chloride in water) |
|
|
Solid Solution Phases |
- solute phase: solid - solvent phase: solid - example: brass (zinc and copper) |
|
|
Entropy |
- tendency for energy to disperse when not constrained - mixing increases entropy |
|
|
Why do solutions form? |
1) entropy 2) intermolecular forces |
|
|
Review of Intermolecular Forces |

|
|
|
What is required for a solution to form? |
- solute/solute and solvent/solvent intermolecular forces must be broken (takes energy, endothermic) - solute/solvent IM forces must be formed (releasing energy, exothermic) |
|
|
(Change in Heat Energy of a Solution)
ΔHsolution = |
= ΔHsolute + ΔHsolvent + ΔHmix |
|
|
ΔHsolution ≈ 0 |
- constant entropy and higher entropy, entropy drives solution formation |
|
|
ΔHsolution < 0 |
- lower energy higher entropy, both drive solution formation |
|
|
ΔHsolution > 0 |
- unless solution is non-ideal, and forms exothermically |
|
|
When are gases soluble in eachother?
a) at STP b) always c) never |
b |
|
|
When will something dissolve? |
- like dissolves like - when solvent and solute structures are similar, solvent molecules will attract the solute particles at least as well as the solute particles are attracted to each other |
|
|
In what kind of solvent are polar molecules and ionic compounds more soluble? |
polar |
|
|
In what kind of solvent are nonpolar molecules more soluble? |
nonpolar |
|
|
(Heat of Hydration)
ΔHsolution = |
= ΔHsolute + ΔHhydration
ΔHhydration: ΔHsolvent + ΔHmix |
|
|
Why is the Heat of Hydration of ions always negative? |
- hydrogen bonds (dipole-dipole) are weak when compared to ion-dipole attractions (more heat is released in ion-water interactions) |
|
|
What happens when ΔHsolute < ΔHhydration? |
- less heat consumed than released - Sign of ΔHsolution is negative - formation of aqueous solution is exothermic and favorable |
|
|
What happens when ΔHsolute > ΔHhydration? |
- more heat consumed than released - Sign of ΔHsolution is positive - formation of aqueous solution is endothermic and unfavorable |
|
|
What happens when ΔHsolute ≈ ΔHhydration? |
- Sign of ΔHsolution is close to 0 (+ or -) - formaiton of aqueous solution is sligtly endo or slightly exothermic |
|
|
Solution Equilibrium |
- when an ionic solute is added to a polar solvent, the ions begin to dissolve (dissolution)
- as solution becomes more concentrated, some of the ions can redeposit as solid (deopsition)
- when rate of dissolution = rate of deposition, equilibrium is reached (or rate of dissolution = rate of vaporization for gaseous solutes in liquid solvents)
- changes with temperature for solid and gaseous solutes
- changes with pressure for gaseous solutes |
|
|
What happens to gas solubility when temperature changes? |
all common gases become less soluble when temperature increases and more soluble when temperature decreases |
|
|
What happens to gas solubility when pressure changes? |
as partial pressure of a gas above a liquid increases, the solubility of the gas increases. |
|
|
Henry's Law says.... |
solubility of a gas in a liquid is directly proportional to the gas pressure |
|
|
Sgas = |
= kH (Pgas)
Sgas: solubility of gas solute (molar concentration, M)
kH: Henry's Law constant (M/atm)
Pgas: partial pressure of gas solute (atm) |
|
|
Molarity |
- M - amount solute (mol)/ vol of solution (L) - units: mol/L |
|
|
Molality |
- m - amount solute (mol)/ mass of solvent (kg) - units: mol/kg |
|
|
Mole Fraction |
- X - amount solute (mol)/ amount solution (mol) - no units |
|
|
Mole Percent |
- mole fraction x 100 |
|
|
Parts by Mass |
- mass of solute/ mass of solution x factor |
|
|
Percent by Mass |
- mass solute/ mass solution x 100 - units: % |
|
|
Part per million by Mass |
- ppm - mass solute/ mass solution x 10^6 |
|
|
Part per billion by Mass |
- ppb - mass solute/ mass solution x 10^9 |
|
|
Parts by Volume |
- vol solute/ vol solution x factor |
|
|
Units of concentration and solution quantities flow chart for review |
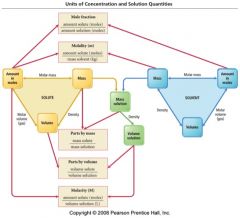
|
|
|
A mixture of H2, N2, and Ar has mole fractions of 0.345 and 0.058 for H2 and N2, respectively. What is XAr?
a) 0.597 b) 0.603 c) 0.900 d) 1.000 e) None of these |
a |
|
|
A solution is prepared by dissolving 15.0 g of NaCl in 0.450 kg of water. The final volume of the solution is 500. mL (density of NaCl = 2.165 g/mL). For this solution, calculate: a) molarity (M) b) molality (m) c) mole fraction (XNaCl) d) mole percent (%) e) percent by mass (%) f) parts per million by mass g) parts per billion by mass h) percent by volume (%) |
a) .513M b) .571 mol/kg c) .0102 d) 1.02% e) 3.23% f) 3.23 x 10^4 ppm g) 3.23 x 10^7 ppb h) 1.39% |
|
|
What is the molality of a solution that is prepared by mixing 30.5 mL of CH3OH (d= 0.792 g/mL) and 387 mL of CH3CH2CH2OH (d= 0.811 g/mL).
a) 0.630 m b) 2.40 m c) 1.57 m d) 2.57 m e) 4.80 m |
b |
|
|
Colligative Properties |
- properties whose value depends only on the number of solute particles, and not on what they are (there are 4 properties) - generally related to the different attractive forces and solute particles occupying solvent molecules positions |
|
|
Vapor Pressure Lowering |
- colligative property - pure solvent reaches a liquid/vapor equilibrium - addition of a nonvolatile solute reduces the rate of vaporization, decreasing the amount of vapor - equilibrium is re-established, but with a smaller number of vapor molecules (therefore the vapor pressure will be lower)
|
|
|
What does adding a non volitile solute to a solution do to the boiling point of the solution? |
raises it |
|
|
ΔTb = |
(change in boiling point) = m∙Kb
m: molality (mol/kg) Kb: boiling point elevation constant (°C) |
|
|
What does adding a non volitile solute to a solution do to the freezing point of the solution? |
lowers it |
|
|
ΔTf = |
(change in freezing point) = m∙Kf
m: molality (mol/kg) Kf: freezing point depression constant (°C)
|
|
|
Illustration of boiling point elevation and freezing point lowering |
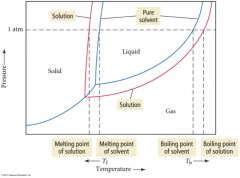
|
|
|
Raoult's Law states.... |
In an ideal solution, each liquid exerts a vapor pressure proportional to its mole fraction times the vapor pressure of the pure liquid |
|
|
PA = |
= (xA)(PA°)
xA: molar fraction (mol) PA°: vapor pressure of pure liquid
|
|
|
Pobs = |
= xAPA° + xBPB° = PA + PB |
|
|
What makes a solution non-idea or real? |
- when particle-particle interactions (solvent/solvent solvent/solute solute/solute) are not similar in magnitude - real solutions deviate from Raoult's Law |
|
|
What happens to real vapor pressure when there are strong solute/solvent interactions? |
it is more difficult for molecules to vaporize (less gas molecules) so the real vp will be lower than the ideal vp |
|
|
What happens to real vapor pressure when there are weak solute/solvent interactions? |
it is less difficult for molecules to vaporize (more gas molecules) so the real vp will be higher than ideal vp |
|
|
Illustration of deviations from Raoult's Law |

|
|
|
How many grams of ethylene glycol (C2H6O2) must be added to 1.0 kg H2O to give a solution that boils at 105 °C? Kb (H2O) = 0.512 °C/m
|
6.1 x 10^2 g of C2H6O2 |
|
|
Osmosis |
the flow of solvent from a solution of low concentration into a solution of high concentration |
|
|
Semi-permeable Membrane |
allows solvent to pass through but not solute |
|
|
Osmotic Pressure |
- amount of pressure needed to keep osmotic flow from taking place - directly proportional to molarity |
|
|
Illustration of Osmotic Pressure |
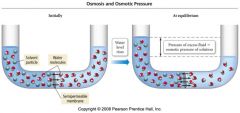
|
|
|
Π = |
= MRT
M: molarity (mol/L) R: .08206 (atm∙L)/(mol∙K) T: temp (K) |
|
|
What is the molar mass of lysozyme if 0.0750 g per 100.0 mL gives an osmotic pressure of 1.32x 10−3 atm at 25 °C? |
5.39 x 10^-6 mol |
|
|
What is the Van't Hoff Factor? |
- ratio of moles of solute particles to moles of formula units dissolved (i)
- measured van’t Hoff factors are generally less than the theoretical due to ion pairing in solution |
|
|
What does the Van't Hoff Factor account for? |
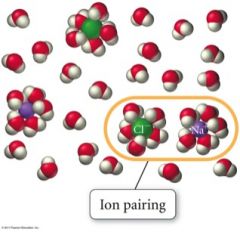
- the fact that ions do not completely dissolve - ionic compounds produce multiple solute particles for each formula unit
|
|
|
What equations require the Van't Hoff Factor for ion solutes? |
- change in boiling point (ΔTb) - change in freezing point (ΔTf) - osmotic pressure (Π) |
|
|
Examples of Van't Hoff Factors at .05m concentration in aqueous solution |
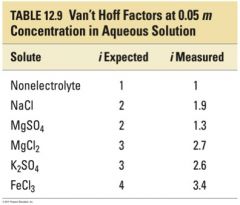
|
|
|
A solution contains 0.102 mol Ca(NO3)2 and 0.927 mol H2O. Calculate the vapor pressure of the solution at 55 ºC.
(Psolution = i∙XH2O∙PºH2O) |
88.8 torr |
|
|
Calculate the theoretical boiling point of a solution made by dissolving 10.0 g of NaCl (MM 58.44g) in 54.0 g of water.
(ΔTb = i x m x Kb) |
103.24°C |
|
|
Isosmotic Solution |
- same osmotic pressure on outside of the cell as the solution inside - no net flow |
|
|
Hyperosmotic Solution |
- higher osmotic pressure on the outside than the solution inside the cell - net flow of water out of the cell causing it to shrivel |
|
|
Hyposmotic Solution |
- lower osmotic pressure on the outside than the solution inside the cell - net flow of water into the cell causing it to swell |
|
|
Illustration of Iso, Hyper, and Hyposmotic Solutions |
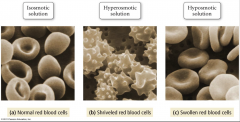
|
|
|
Identify for the following: what kind of mixure?
a) solution b) suspension c) colloid |
a) solution: homogeneous mixture b) suspension: heterogeneous mixture (separates after standing) c) colloid: heterogeneous (does not separate) |
|
|
Colloids |
- particles can coagulate - cannot pass through semi-permeable membrane - hydrophilic (stabilized by attraction for water) - hydrophobic (stabilized by charged surface repulsions) |
|
|
Brownian Motion |
- the random motion of particles suspended in a fluid resulting from their collision with other atoms or molecules in the gas or liquid |
|
|
Tyndall Effect |

- light particles passing through a colloid can be seen because they interact with particles in the colloid |
|
|
How does soap work? |
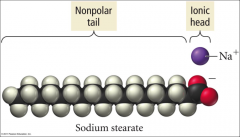
- polar, hydrophillic head interacts with water - nonpolar, hydrophobic tail interacts with grime |

