![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
The force holding two atoms together is called |
Chemical bonds |
|
|
Covalent bonds occur between two |
Nonmetals |
|
|
Ionic bonds occur between |
A metal and non-metal |
|
|
, in covalent bonds atoms______electrons |
Share |
|
|
In an ionic bond there is a________ of electrons from one atom to another |
Transfer |
|
|
A covalent bond that consists of one pair of electrons shared between atoms |
Single |
|
|
A covalent bond that consists of two pairs of electrons shared between atoms |
Double bonds |
|
|
A covalent bond that consists of three pairs of electrons shared between atoms |
Triple bonds |
|
|
Ionic charge of Group 1 elements is |
+1 |
|
|
The ionic charge of group 2 elements is |
+2 |
|
|
Aluminum has an ionic charge of what |
+3 |
|
|
Ionic charge of group 16 is |
-2 |
|
|
The ionic charge of group 17 is |
-1 |
|
|
Transition metals have of irony of charges when the transition metals you need to include what to represent the charge of the metal |
A Roman numeral |
|
|
What does the electron-dot structures represent |
The number of valence electrons |
|
|
How many valence electrons are associated with each groups |
1 1 2 2 13 3 14 4 15 5 16 6 17 7 18 8 |
|
|
What are the 7 diatomic molecules |
N2 O2 F2 CL2 BR2 I2 H2 |
|
|
What are the five binary acids |
HCl hydrochloric acid HF. Hydrofloric acid HBr hydrobromie acid HI hydroponic acid H2S. Hydrosulfuric acid |
|
|
The polyatomic ion the end with -ate the acid should end with what |
-ic |
|
|
It's a polyatomic ends with - ite the acid should end with |
-ous |
|
|
When I mean tertiary acids do you include the prefix Hydro |
No |
|
|
When naming binary acids include the prefix Hydro |
Yes |
|
|
Products on what side of the equation |
Right |
|
|
Reactants are on the what side of the equation |
Left |
|
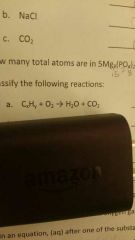
|
Combustión |
|
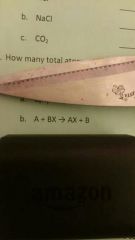
|
Single replacement |
|

|
Double replacement |
|

|
Synthesis |
|

|
Decomposition |
|
|
To determine if a single replacement reaction to occur refer |
To an activity series |
|
|
To determine if a double placement reaction will occur referred to |
Solilbuilty Table |
|
|
What are spectator ions |
Ions that don't do anything |

