![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
A chemical formula gives us the _____ (including the ratios) in a _____ _____.
|
elements
pure substance |
|
|
The elements are listed as their symbols, and the ratios are given as _____ following each element.
|
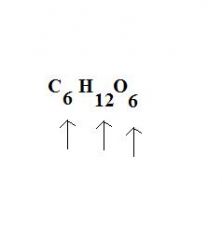
subscripts
|
|
|
Sucrose is a compound composed of twelve carbon atoms, twenty two hydrogen atoms, and eleven oxygen atoms. Write out the formula.
|
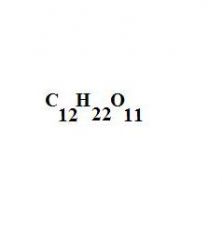
see the picture
|
|
|
If the ratio (the subscripts) of a compound is changed, what happens?
|

Something different is created (i.e. it is no longer the original compound)
|
|
|
What two types of bonds join compounds together?
|
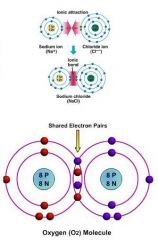
Ionic and Covalent
|
|
|
_____ compounds are formed when elements in ionic form are attracted by electrostatic charges.
|
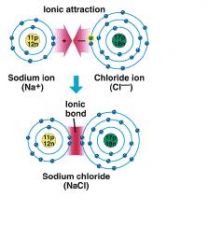
Ionic
|
|
|
_____ compounds are formed when atoms share electrons.
|
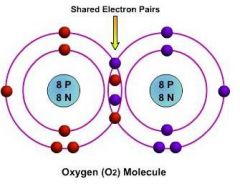
Covalent
|
|
|
_____ _____ are ions (things with a charge) that are composed of multiple atoms that act as a single unit.
|
Polyatomic Ions
|
|
|
When we need to play around with molecules (adjust the quantities), the only option is to adjust the _____.
|

coefficient (the value that precedes the chemical formula)
|
|

see the picture
|
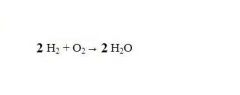
see the picture
|
|

see the picture
|
left side = reactants or reagents
right side = products |
|
|
What is the formula for an ammonium ion?
|
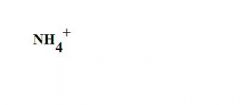
see the picture
|
|
|
What is the formula for a nitrate ion?
|
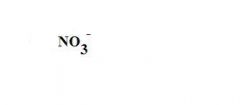
see the picture
|
|
|
What is the formula for a nitrite ion?
|
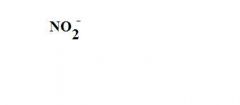
see the picture
|
|
|
What is the formula for an acetate ion?
|
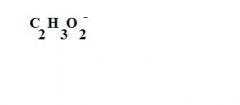
see the picture
|
|
|
What is the formula for a sulfate ion?
|

see the picture
|
|
|
What is the formula for a sulfite ion?
|

see the picture
|
|
|
What is the formula for a phosphate ion?
|

see the picture
|
|
|
What is the formula for a perchlorate ion?
|

see the picture
|
|
|
What is the formula for a carbonate ion?
|
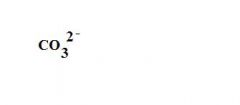
see the picture
|
|
|
What is the formula for a bicarbonate ion?
|
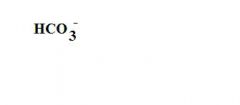
see the picture
|
|
|
What is the formula of a hydroxide ion?
|

see the picture
|
|
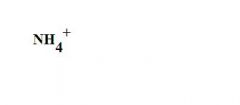
What is this?
|
an ammonium ion
|
|
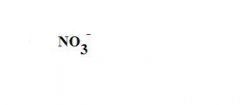
What is this?
|
a nitrate ion
|
|
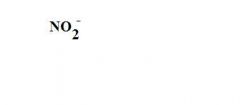
What is this?
|
a nitrite ion
|
|
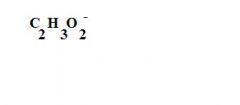
What is this?
|
an acetate ion
|
|

What is this?
|
a sulfate ion
|
|

What is this?
|
a sulfite ion
|
|

What is this?
|
a phosphate ion
|
|

What is this?
|
a perchlorate ion
|
|
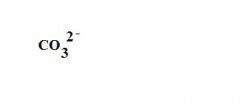
What is this?
|
a carbonate ion
|
|
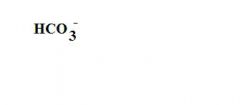
What is this?
|
a bicarbonate ion
|
|

What is this?
|
a hydroxide ion
|

