![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Define atom.
|
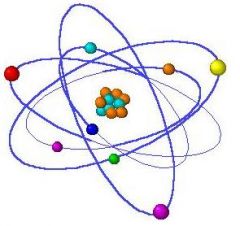
It is the smallest particle that retains the properties of its elements.
|
|
|
Atoms are composed of three subatomic particles. Name them.
|
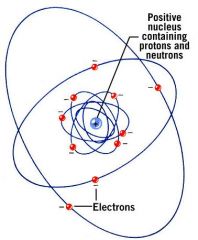
Protons
Neutrons Electrons |
|
|
The _____ is at the center of the each atom.
|
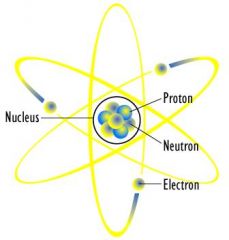
nucleus
|
|
|
What is contained in the nucleus?
|
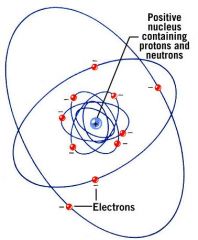
Protons and Neutrons
|
|
|
Where in each atom are the electrons located?
|
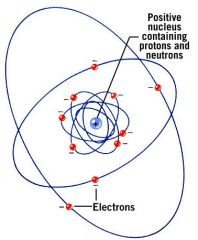
Outside the nucleus in their orbits
|
|
|
What determines the total volume of an atom?
|
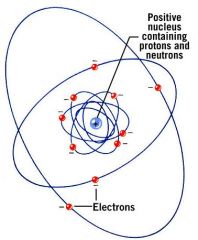
the orbits of the electrons
|
|
|
What is the relative mass of a proton?
|
1
|
|
|
What is the relative charge of a proton?
|
+1
|
|
|
What is the relative mass of a neutron?
|
1
|
|
|
What is the relative charge of a neutron?
|
0
|
|
|
What is the relative mass of an electron?
|
0
|
|
|
What is the relative charge of an electron?
|
-1
|
|
|
What subatomic particle determines the element?
|
the protons
|
|
|
_____ _____ is displayed as a whole number on the periodic table and determines what element you have.
|
atomic number
|
|
|
If the number of electrons in an atom is equal to the number of protons, then the charge is _____.
|
neutral
|
|
|
If the number of electrons in an atom is > the number of protons, then the charge is _____.
|
negative
|
|
|
If the number of electrons in an atom is < the number of protons, then the charge is _____.
|
positive
|
|
|
If an atom has a negative charge , then it is called a/an _____.
|
anion
|
|
|
If an atom has a positive charge , then it is called a/an _____.
|
cation
|
|
|
Fill in the missing information:
Element = sodium Atomic # = ? # of protons = ? # of electrons = ? Charge = ? Ionic Symbol = ? |
Element = sodium
Atomic # = 11 # of protons = 11 # of electrons = 10 Charge = +1 + Ionic Symbol = Na NOTE: Remember, in its elemental form sodium is always an ion. |
|
|
Fill in the missing information:
Element = ? Atomic # = 16 # of protons = ? # of electrons = ? Charge = ? Ionic Symbol = ? |
Fill in the missing information:
Element = sulfur Atomic # = 16 # of protons = 16 # of electrons = 18 Charge = -2 2- Ionic Symbol = S NOTE: Remember, in elemental form, sulfur is always an ion. |
|
|
Fill in the missing information:
Element = ? Atomic # = ? # of protons = 20 # of electrons = ? Charge = ? Ionic Symbol = ? |
Element = calcium
Atomic # = 20 # of protons = 20 # of electrons = 18 Charge = +2 2+ Ionic Symbol = Ca NOTE: Remember, in elemental form, calcium is always an ion. |
|
|
Fill in the missing information:
Element = ? Atomic # = ? # of protons = ? # of electrons = ? Charge = ? 3- Ionic Symbol = P |
Element = ?
Atomic # = 15 # of protons = 15 # of electrons = 18 Charge = -3 3- Ionic Symbol = P NOTE: Remember, in elemental form, phosphorus is always an ion. |
|
|
Define mass number.
|
Neutrons + Protons
|
|
|
What are isotopes?
|
of the same element with different mass numbers/# of neutrons
|
|
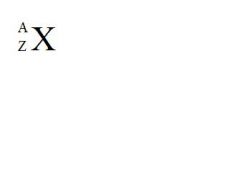
Using this shorthand way of describing an element, what do the "A", the "X", and the "Z" stand for (see the picture)?
|
A = atomic mass (# of protons + neutrons)
X = element symbol Z = atomic number (# of protons) |
|
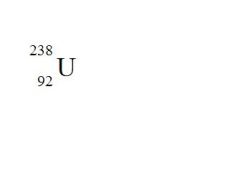
State the element, the atomic mass, and the atomic number for the following (see the picture):
|
element = Uranium
atomic mass = 238 atomic number = 92 |
|
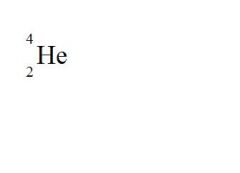
State the element, the atomic mass, and the atomic number for the following (see picture):
|
element = Helium
atomic mass = 4 atomic number = 2 |
|
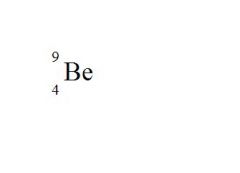
State the element, the atomic mass, and the atomic number for the following (see picture):
|
element = Beryllium
atomic mass = 9 atomic number = 4 |
|
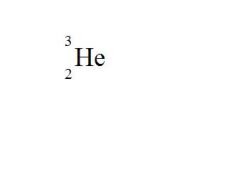
State the element, the atomic mass, and the atomic number for the following:
|
element = Helium
atomic mass = 3 atomic number = 2 |

