![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
211 Cards in this Set
- Front
- Back
|
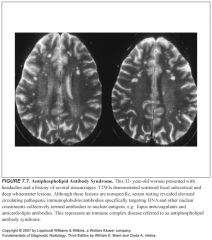
Two broad categories of white matter disease:
|
Demyelinating
Dysmyelinating |
|
|
What’s the difference between demyelination and dysmyelination?
|
-Demyelination is an acquired disorder that affects normal myelin
-Dysmyelination is an inherited disorder affecting the formation or maintenance of myelin -Dysmyelination is rare |
|
|
Why is demyelination seen in MS?
|
Because there are abnormal immunoglobulins that activate T cells against myelin
|
|
|
Average age of onset of MS?
|
20-40
|
|
|
Gender predominance in MS?
|
Female: male= 2:1
|
|
|
Classic definition of MS?
|
Multiple CNS lesions separated in both time and space
|
|
|
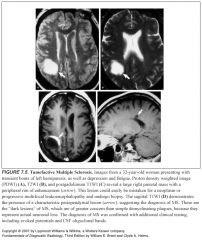
What do active MS lesions look like on path?
|
-Areas of selective destruction of myelin sheaths and perivenular inflammation with relative sparing of the underlying axons.
-The inflammation is a key differentiating feature between MS and other white matter conditions (e.g., central pontine and extrapontine myelinolysis and PRES) |
|
|
Is imaging diagnostic of MS?
|
NO
|
|
|
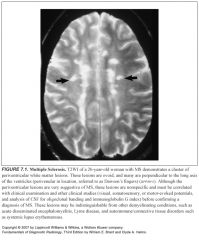
Which sequence is best for finding MS lesions?
|
T2 and FLAIR
|
|
|
Why is FLAIR so helpful in evaluation of possible MS lesions?
|
It provides heavy T2 weighting while suppressing signal from CSF, so periventricular lesions show up better
|
|
|
Disadvantage of the FLAIR sequence?
|
It has mild limitations in the posterior fossa and spine, partly due to pulsation artifacts
|
|
|
Where are MS lesions typically located?
|
Periventricular or subcortical
|
|
|
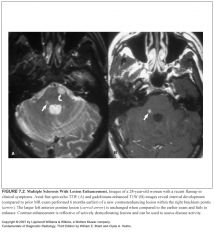
What does it mean if an MS lesions enhances?
|
That it’s a new lesion with active demyelination and disruption of the blood-brain barrier
|
|
|
What are T2 bright non-enhancing lesions thought to represent?
|
-Scarring
-This is a focal proliferation of astroglia at the site of injury (i.e., gliosis) |
|
|
What are “dark” lesions of MS?
|
These are seen in severe cases of MS and represent actual loss of neuronal tissue rather than simple demyelination
|
|
|
What is seen in chronic MS?
|
Diffuse loss of deep cerebral white matter, with associated thinning of the corpus callosum and potential ex vacuo ventriculomegaly
|
|
|
What types of patient’s have a similar appearance and symptoms to MS?
|
Patients with migraines
|
|
|
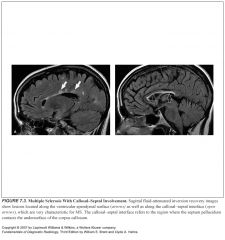
What pattern of lesions is suggestive of MS?
|
-Periventricular lesions that are ovoid and aligned perpendicular to the long axis of the ventricles
-Lesions along the callosal septal interface -Lesions that are confluent in nature and greater than 6 mm in diameter with a periventricular location |
|
|
What percent of spinal cord MS lesions are associated with plaques in the brain?
|
70-80%
|
|
|
What do spinal lesions of MS look like?
|
They can have mild mass effect as well as contrast enhancement, thus mimicking a tumor.
|
|
|
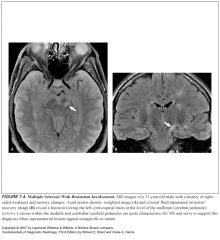
Where should you look for MS lesions in pts older than 50 years old (when it’s confusing to tell between ischemia and MS)?
|
Places where ischemic changes are rare:
-Cerebellar and cerebral peduncles -Corpus callosum -Medulla -Spinal cord |
|
|
MS lesions can also present as a large, conglomerate deep white matter mass that can be mistaken for a neoplasm. How can you tell that it’s MS and not a tumor?
|
-There is often a peripheral crescentic rim of contrast enhancement which represents the advancing region of active demyelination.
-Lack of significant mass effect |
|
|
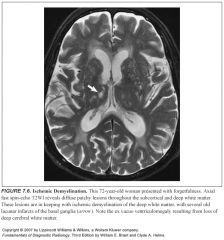
Why is the deep white matter more susceptible to ischemic injury?
|
Because it is supplied by long, small caliber penetrating end arteries without significant collateral supply
|
|
|
Why do deep penetrating vessels become narrowed and cause ischemia demyelination?
|
Arteriosclerosis and lipohyalin deposits
|
|
|
Which areas of the brain are usually spared from ischemic demyelination?
|
-Cortex
-Subcortical U fibers -Central corpus callosum -Medulla -Midbrain -Cerebellar peduncles |
|
|
What do ischemic white matter lesions look like on histo?
|
Axonal atrophy with diminished myelin
|
|
|
What’s another name for multi-infarct dementia?
|
Binswager disease
|
|
|
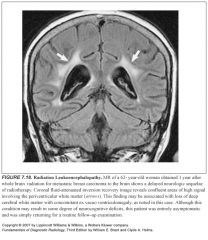
Other names for leukoariosis?
|
-Microangiopathic leukoencephalopathy
-Subcortical arteriosclerotic encephalopathy |
|
|
What percent of patients presenting with MS are older than 50?
|
10%
|
|
|
What areas are spared in LA but not in MS?
|
The subcortical arcuate fibers
Corpus callosum |
|
|
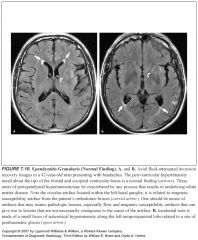
What is ependymitis granularis?
|
-Area of high signal on a T2 along the tips of the frontal horns
-Normal anatomic findings that may mimic pathology -Relates to porous ependyma |
|
|
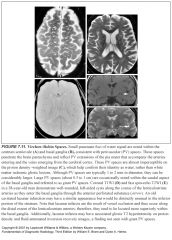
What are Virchow-Robin spaces?
|
-CSF filled perivascular clefts
-Foci of high signal on T2 |
|
|
Where are prominent pericascular spaces usually located?
|
-Centrum semiovale (high cerebral hemispheric white matter)
-Lower basal ganglia at the level of the anterior commissure -Where the lenticulostriate arteries enter the brain parenchyma |
|
|
How can you tell a dilated perivascular space from a parenchymal lesion?
|
-Use proton density or FLAIR images
-On these images, Fluid is attenuated, and CSF has similar signal intensity as white matter. So, perivascular spaces will look isointense to brain while ischemic lesions will be bright (due to associated gliosis) |
|
|
How does location help you distinguish a perivascular space from a parenchymal lesion?
|
-Lacunar infarcts are usually in the upper 2/3rds of the basal ganglia
-Periventricular spaces are often symmetric within the inferior third of the striatum (think about there being water standing in the bottom of the basal ganglia) |
|
|
What virus is associated with PML?
|
The JC virus (Jesus Christ! I’ve got PML!)
|
|
|
Which pts get PML?
|
-Severely immunocompromised pts
-Those with AIDS, organ transplants, lymphoma |
|
|
Which cells does the JC virus infect?
|
The oligodendrocytes
|
|
|
What do oligodendrocytes do?
|
-They generate the myelin sheath
-So damage results in widespread demyelination |
|
|
What parts of the brain are typically affected in PML?
|
-The deep cerebral white matter with subcortical U fiber involvement
-Typically, the parietooccipital region is affected -The cortex and deep gray matter are spared |
|
|
Characterize PML lesions:
|
-Lack of mass effect
-Contrast enhancement -Hemorrhage -These lesions progress rapidly and coalesce into larger confluent asymmetric areas |
|
|
What cells are affected in HIV encephalopathy?
|
-The microglia (brain macrophages)
-The cytokines and excitatory compounds that are produced as a result of this infection have a toxic effect on adjacent neurons |
|
|
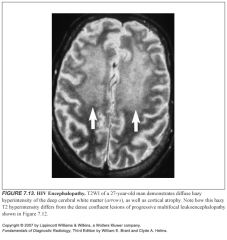
What does HIV encephalopathy look like?
|
-Mild cerebral atrophy without focal abnormality is the most common appearance
-Occasionally, there are focal or diffuse white matter hyperintensities on T2 which are subtle -Often bilateral and relatively symmetric |
|
|
So how can you tell HIV encephalopathy apart from PML?
|
-The supratentorial white matter signal is ill defined in HIV encephalopathy and often involves a large area, in contrast to the dense lesions of PML
-HIV lesions do not demonstrate enhancement |
|
|
What does ADEM stand for?
|
Acute disseminated encephalomyelitis
|
|
|
Describe the pathogenesis of ADEM:
|
-It’s a postinfectious and postvaccinal encephalomyelitis
-Typically occurs after a viral illness or vaccination, with measles, rubella, varicella, and mumps being the most common agents -Sometimes no antecedent infection or inciting malady is identified -The body’s antiviral immune reaction cross-reacts with myelin sheaths, resulting in an acute, aggressive form of demyelination -Most resolve spontaneously, but up to 25% can have permanent neurologic sequelae |
|
|
What percent of ADEM pts have permanent neurologic sequelae?
|
up to 25%
|
|
|
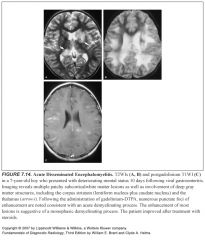
What does ADEM look like?
|
-Multifocal or confluent white matter lesions similar to MS
-Gray matter can also be affected |
|
|
How can you tell ADEM apart from MS?
|
-MS has a relapsing and remitting course, while ADEM is monophasic
-If the majority of the lesions enhance, this is suggestive of a monophasic disease (ADEM) |
|
|
What does SSPE stand for?
|
Subacute sclerosing panencephalitis
|
|
|
What is it?
|
-A slowly progressive infection caused by the measles virus
-Variable course |
|
|
Who gets SSPE?
|
Children between the ages of 5 and 12 years who have had measles, usually before the age of 3
|
|
|
What does SSPE look like?
|
Patchy areas of periventricular demyelination as well as lesions of the basal ganglia
|
|
|
What is the most common fatal encephalitis?
|
Herpes encephalitis
|
|
|
What things are most commonly associated with ADEM?
|
Measles, rubella, varicella, and mumps are the most common agents
|
|
|
Presenting signs of herpes encephalitis?
|
-Headache, fever, mental deterioration, seizures
-Variable presentation makes diagnosis difficult, making the radiologist very important in dx if there are suggestive imaging findings |
|
|
How is the dx of herpes encephalitis confirmed?
|
-Finding herpes DNA in the CSF on PCR
-This test takes a while to come back |
|
|
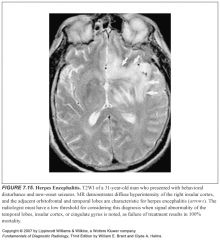
Where does HSV like to go in the brain?
|
The limbic system, with localization of infection to the temporal lobes, insular cortex, subfrontal area, and cingulate gyri
|
|
|
What does HSV encephalitis look like?
|
-T2 hyperintensity of the involved structures
-Variable cotnrast enhancement -Initially it’s unilateral, but sequential bilateral involvement is highly suggestive -Hemorrhage within involved parenchyma is strongly suggestive |
|
|
Who gets central pontine myelinolysis?
|
-Usually in malnourished patients
-Associated with rapid correction of electrolyte abnormalities, particularly hyponatremia |
|
|
Signs and symptoms of CPM?
|
-Rapidly evolving corticospinal syndrome
-Quadriplegia, acute mental status changes -Locked in state -Poor prognosis |
|
|
Which cells are affected in CPM?
|
The oligodendroglial cells
|
|
|
What’s another name for CPM?
|
Osmotic demyelination syndrome *very descriptive*
|
|
|
Which areas are affected by CPM?
|
-Central areas (hence the name)
-Central pons, central thalamus -Globus pallidus, putamen, lateral geniculate body Extrapontine sites have been described (extrapontine myelinolysis) -White matter of the cerebellum, thalamus, globus pallidus, putamen, lateral geniculate body |
|
|
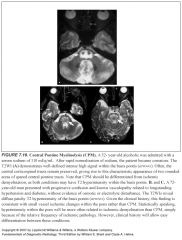
What does CPM look like?
|
-High T2 signal in the affected regions
-No inflammatory reaction (no enhancement) |
|
|
What distinguishes CPM from MS?
|
No enhancement (no inflammatory reaction, just plain ole’ rupture of the myelin sheaths)
|
|
|
What does PRES stand for?
|
Posterior reversible encephalopathy syndrome
|
|
|
How do patients with PRES present?
|
Headache, seizures, visual changes, altered mental status
|
|
|
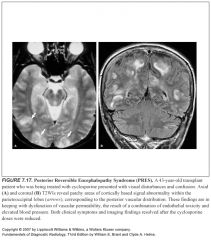
What does PRES look like?
|
-Symmetric areas of bilateral subcortical and cortical vasogenic edema within the parietooccipital lobes
-Changes primarily involve the posterior vascular distribution |
|
|
Why does PRES happen?
|
-Temporary failure of the autoregulatory capabilities of the cerebral vessels, leading to hypoperfusion, breakdown of the blood-brain barrier and consequent vasogenic edema, but no acute ischemic changes
-There’s some kind of endothelial injury that occurs, increasing capillary permeability |
|
|
Why does the autoregulation of cerebral perfusion fail in PRES?
|
-Usually it maintains constant blood flow to the brain
-This can be overcome a “breakthrough” point when the increased systemic blood pressure is transmitted to the brain, resulting in hyperperfusion -The increased perfusion pressure is sufficient to overcome the blood-brain barrier, allowing extravasation of fluid, macomolecules, and even red blood cells into the brain parenchyma |
|
|
Why is the posterior circulation more involved?
|
It’s related to the relatively poor sympathetic innervation of the posterior circulation
|
|
|
What things are associated with PRES?
|
-Treatment with cyclosporin A or tracolimus (FK506)
-Acute renal failure/uremia -HUS -Eclampsia -TTP -Lots of chemo agents, including interferon HTN is not universally present, especially in the setting of immunosuppression |
|
|
Is PRES always reversible?
|
PRES is not always reversible and may occasionally result in hemorrhagic infarctions.
|
|
|
Who gets Marchiafava-Bignami disease?
|
-Usually alcoholics
-First described in Italian red wine drinkers |
|
|
What is it?
|
-Felt to represent a form of osmotic demyelination
-Onset is usually insidious, with the most common symptom being nonspecific dementia |
|
|
What areas of the brain are affected in Marchiafava-Bignami disease?
|
-The central fibers (medial zone) of the corpus callsoum primarily
-Other white matter tracts may be involved, including the anterior and posterior commissures, the centrum semiovale, and the middle cerebral peduncles |
|
|
What vitamin is deficient in Wernicke’s and Korsakoff’s?
|
B1, thiamine
|
|
|
Which patients get thiamine deficiency?
|
-Alcoholics
-Hematologic malignancies -Pregnant patients with hyperemesis gravidarum |
|
|
Clinical triad of Wernicke’s encephalopathy?
|
-Acute ocular movement abnormalities
-Ataxia -Confusion |
|
|
What do you add to Wernicke's to make if Wernicke-Korsakoff’s?
|
Persistent learning and memory deficits
|
|
|
What does Wernicke-Korsakoff’s encephalopathy look like?
|
-Acutely, areas of T2 hyperintensity and enhancement of the mamillary bodies, basal ganglia, thalamus, brainstem, periaqueductal involvement.
-Chronically, there’s atrophy of the mamillary bodies, midbrain tegmentum, and dilation of the 3rd ventricle. |
|
|
Is PRES always reversible?
|
PRES is not always reversible and may occasionally result in hemorrhagic infarctions.
|
|
|
Who gets Marchiafava-Bignami disease?
|
-Usually alcoholics
-First described in Italian red wine drinkers |
|
|
What is it?
|
-Felt to represent a form of osmotic demyelination
-Onset is usually insidious, with the most common symptom being nonspecific dementia |
|
|
What areas of the brain are affected in Marchiafava-Bignami disease?
|
-The central fibers (medial zone) of the corpus callsoum primarily
-Other white matter tracts may be involved, including the anterior and posterior commissures, the centrum semiovale, and the middle cerebral peduncles |
|
|
What vitamin is deficient in Wernicke’s and Korsakoff’s?
|
B1, thiamine
|
|
|
Which patients get thiamine deficiency?
|
-Alcoholics
-Hematologic malignancies -Pregnant patients with hyperemesis gravidarum |
|
|
Clinical triad of Wernicke’s encephalopathy?
|
-Acute ocular movement abnormalities
-Ataxia -Confusion |
|
|
What do you add to Wernicke's to make if Wernicke-Korsakoff’s?
|
Persistent learning and memory deficits
|
|
|
What does Wernicke-Korsakoff’s encephalopathy look like?
|
-Acutely, areas of T2 hyperintensity and enhancement of the mamillary bodies, basal ganglia, thalamus, brainstem, periaqueductal involvement.
-Chronically, there’s atrophy of the mamillary bodies, midbrain tegmentum, and dilation of the 3rd ventricle. |
|
|
What disease does chronic Wernicke-Korsakoff look exactly like?
|
Leigh disease
|
|
|
Types of radiation damage the brain?
|
-Radiation leukoencephalopathy (an induced vasculopathy)
-Radiation necrosis and radiation arteritis (more serious) |
|
|
What cumulative dose must be met for radiation leukoencephalitis to occur?
|
40 Gy
|
|
|
How long does it take for radiation leukoencephalitis to occur?
|
6-9 months after treatment
|
|
|
What’s the difference between radiation therapy and gamma knife?
|
-Gamma knife is an ablative procedure designed to destroy target tissue, so it is more likely to incite frank radiation necrosis
-Radiation therapy is not ablative |
|
|
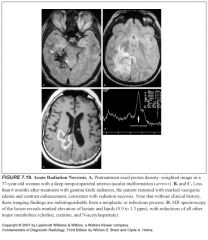
When does radiation necrosis typically present?
|
Between 6 and 24 months after treatment
|
|
|
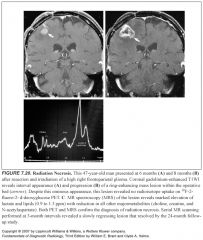
What does radiation necrosis look like?
|
-Enhancing lesion with mass effect and ring enhancement
-Multiple foci of enhancement -Telangiectasia within the field can be seen |
|
|
How can you tell the difference between radiation necrosis and tumor recurrence?
|
Sometimes you can’t
-If the lesion regresses, it’s radiation necrosis -PET and MR spectroscopy are valuable |
|
|
What would tumor recurrence look like on PET?
|
-High FDG uptake
-Radiation necrosis would not be hypermetabolic |
|
|
What are the brain metabolites analyzed on MR spectroscopy?
|
Choline, creatine, N-acetylaspartate (NAA), lactate
|
|
|
So what do you think about if you see an elevated choline spike?
|
-Well, choline reflects cellular density because there’s lots of choline in cell walls
-If it’s elevated, that can indicate tumor |
|
|
How is creatine used?
|
-It is a normal metabolite and is often stable in a variety of disease conditions
-So it's used as a denominator in calculating the choline/Cr ratio or the NAA/Cr ratio to correct for individual variation and to allow for comparison between individual subjects |
|
|
What does the NAA spike indicate?
|
-NAA is a neuronal marker (good thing they both start with N)
-Its spike will be lost in neuronal loss or damage (radiation necrosis , MS) |
|
|
What happens to blood vessels in the radiation field?
|
-They can undergo endothelial hypertrophy, medial hyalinization, and fibrosis
-This can result in progressive vascular narrowing that may be obliterative |
|
|
Which vessels are more often affected by radiation changes?
|
Cavernous and supraclinoid carotid arteries
|
|
|
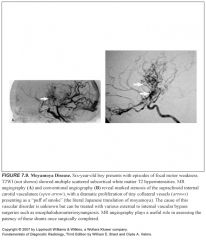
How is radiation damage related to Moyamoya?
|
If radiation causes these arteries to be narrowed, compensatory lenticulostriate collaterals can proliferate, causing a moyamoya appearance on angiography
|
|
|
In which patients was moyamoya classically described in?
|
Children with idiopathic disease
|
|
|
Which chemo agent is syngergistic with radiation in causing white matter changes?
Why? |
Methotrexate
Radiation alters the BBB, allowing increased penetration of methotrexate to neurotoxic levels |
|
|
Name the two conditions seen with the methotrexate/radiation combo:
|
-Mineralizing microangiopathy
-Necrotizing leukoencephalopathy |
|
|
Which patients are most frequently affected by the methotrexate/radiation combo?
|
Children being treated for leukemia
|
|
|
Describe mineralizing microangiopathy:
|
-Seen in up to one third of children on the nasty MTX/radiation combo
-Diffuse destructive changes -Symmetric corticomedullary junction and basal ganglia calcifications -Diffuse signal abnormality throughout the white matter |
|
|
Describe necrotizing leukoencephalopathy:
|
-Caused by the nasty methotrexate/radiation combo
-Widespread damage to the white matter consiting of demyelination, necrosis, and gliosis -MR reveals diffuse, large, confluent areas of white matter signal abnormality with cortical sparing |
|
|
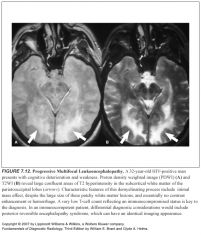
Define dysmyelination:
|
-Conditions are referred to as the leukodystrophies
-Myelin is abnormally formed or cannot be maintained in a normal state because of an inherited enzymatic or metabolic disorder |
|
|
How can you tell the different dysmyelination syndromes apart?
|
-These conditions look very similar with a few distinguishing features
-Factors that are helpful in differentiation between the leukodystrophies include age of onset and pattern of white matter involvement |
|
|
What is the most common of the leukodystrophies?
|
Metachromatic leukodystrophy
|
|
|
What enzyme is missing in Metachromatic leukodystrophy?
|
Arylsulfatase A
|
|
|
When does metachromatic leukodystrophy present?
|
Infantile form: 1-2 years (more common)
Juvenile form: 5-7 years |
|
|
Head size in metachromatic leukodystrophy?
|
Normal
|
|
|
What does metachromatic leukodystrophy look like?
|
-Diffuse symmetric white matter involvement
-Sparing of the subcortical U fibers -No gray matter involvement |
|
|
Why does adrenal leukodystrophy contain “adrenal” in its name?
|
Because pts have symptoms related to the adrenal gland such as adrenal insufficiency or abnormal skin pigmentation
|
|
|
How is adrenal leukodystrophy transmitted?
|
X-linked recessive
|
|
|
Which enzyme is missing in adrenal leukodystrophy?
|
Peroxisomal enzyme
|
|
|
Age of onset in adrenal leukodystrophy?
|
5-10 years
|
|
|
Head circumference in adrenal leukodystrophy?
|
Normal
|
|
|
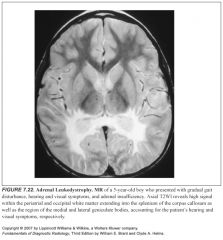
What does adrenal leukodystrophy look like?
|
-Striking predilection for the visual and auditory pathways
-Presents with symmetric involvement of the periatrial white matter with extension into the splenium of the corpus callosum -There is early extension into the medial and lateral geniculate nuclei (these are parts of the auditory and visual pathways, respectively) |
|
|
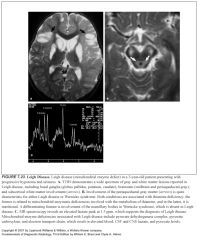
What is Leigh disease?
|
A mitochondrial enzyme defect
|
|
|
When does Leigh disease present?
|
Infancy or early childhood, younger than 5
|
|
|
What does Leigh disease look like?
|
-Exactly like Wernicke’s encephalopathy BUT mamillary bodies are spared!
-Focal necrotic lesions in the basal ganglia and thalamus as well as in the subcortical white matter -Lesions may also extend into the midbrain, medulla, and posterior columns of the spinal cord -Characteristic finding: involvement of the periaqueductal gray matter |
|
|
What are the mitochondrial disorders?
|
-Leigh disease
-MERRF (myoclonic epilepsy and ragged-red fibers) -MELAS (mitochondrial myelopathy, encephalopathy, lactic acidosis, and strokelike episodes) |
|
|
Which leukodystrophies are associated with a big head?
|
-Alexander and Canavan diseases
-They’re rare |
|
|
When do Alexander and Canavan disease present?
|
At less than one year, often in the first weeks
|
|
|
What does Alexander disease look like?
|
-White matter lesions often begin in the frontal white matter and progress posteriorly (like Alexander the Great, marching across the brain)
-Gray matter NOT involved |
|
|
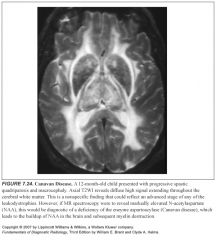
What enzyme is missing in Canavan disease?
|
-Aspartoacylase
-This leads to buildup of NAA in the brain with subsequent myelin destruction |
|
|
What is seen on spectroscopy in Canavan disease?
|
Ginormous NAA peak
|
|
|
What disease is associated with a ginormous NAA peak?
|
Canavan disease
|
|
|
Where is CSF produced?
|
In the choroid plexus of the lateral, third, and fourth ventricles
|
|
|
How is CSF reabsorbed?
|
-Primarily through the arachnoid villi, which project into the dural sinuses
-A significant amount may be reabsorbed via the ependymal lining of the ventricles (this becomes important when the ventricles are obstructed) |
|
|
Describe how CSF flows through the ventricular system:
|
From the lateral ventricles
Through the foramen of Monro Through the third ventricle Through the cerebral aqueduct -Through the fourth ventricle -Then it leaves the ventricular system via the foramina of Luschka and Magenie |
|
|
What happens to CSF after it leaves the ventricular system?
|
It travels through the basilar cisterns and over the surfaces of the cerebral hemispheres
|
|
|
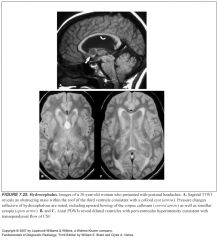
Define non-communicating hydrocephalus:
|
Caused by an obstruction within the ventricular system
|
|
|
Define communicating hydrocephalus:
|
-Obstruction is outside the ventricular system, located instead within the arachnoid space
-CSF is able to exit the ventricular system, but fails to undergo normal resorption by the arachnoid villi |
|
|
Which part of the ventricular system doesn’t dilate much?
Why? Why is this important? |
-The fourth ventricle,
-Because the posterior fossa is a relatively confined space -So, it can’t be reliably used to distinguish communicating vs. non-communicating hydrocephalus. |
|
|
Where should you really pay attention when assessing for hydrocephalus?
|
-The third ventricle and the temporal horns
-The temporal horns are the most sensitive place to look -Convex bowing of the lateral walls and the inferior recess of the third ventricle is classic -Also, look for bowing and stretching of the corpus callosum on sagittal images |
|
|
How can you tell the difference between hydrocephalus and atrophy?
|
-Look at the third ventricle and temporal horns
-These should not be dilated with atrophy, and they are surrounded by tissue that is not typically affected by significant atrophy -Also, look at the sulci, they should be proportionally atrophied relative to the ventricles. Otherwise, it’s hydrocephalus. |
|
|
How does CSF leave the ventricular system?
|
Via the foramina of Luschka and Magenie
|
|
|
Most common causes of acute hydrocephalus?
|
-Subarachnoid hemorrhage
-Meningitis |
|
|
What kind of hydrocephalus do subarachnoid hemorrhage and meningitis cause?
|
-Communicating and/or non-communicating
-Obstruction can occur at any level within the ventricular system, basilar cistern, or the arachnoid villi |
|
|
Why would subarachnoid hemorrhage and meningitis cause obstructive hydrocephalus?
|
-Adhesions and inflammation are thought to cause the obstruction
-No obstructing mass is typically found |
|
|
What causes congenital aqueductal stenosis?
|
Benign congenital webs may form across the cerebral aqueduct
|
|
|
What is thought to cause the Chiari and Dandy-Walker malformations?
|
The Chiari and Dandy-Walker malformations are believed to represent adhesions occuring during CNS development at the outlet of the fourth ventricle and posterior fossa
|
|
|
Where do colloid cysts typically cause obstruction?
|
The anterior third ventricle, at the foramen of Monro
|
|
|
What tumors typically obstruct the cerebral aqueduct?
|
Pineal tumors and tectal gliomas
|
|
|
What tumors typically obstruct the fourth ventricle?
|
Ependymomas and medulloblastomas
|
|
|
Which spot should always be scrutinized when evaluating for hydrocephlus?
|
The aqueduct, if you don’t see a pulsatile flow void, aqueductal stenosis should be considered
|
|
|
What tumors typically obstruct the foramen of Monro?
|
Colloid cysts
|
|
|
What are signs of acute hydrocephalus?
|
There should be a striking amount of transependymal CSF flow causing a dramatic high signal in the periventricular white matter on T2
|
|
|
What are signs of chronic hydrocephalus?
|
The degree of transependymal flow is minimal
|
|
|
Classic triad of symptoms in normal pressure hydrocephalus?
|
Dementia, incontinence, ataxia
|
|
|
What is normal pressure hydrocephalus?
|
-Chronic low level hydrocephalus
-A slight gradient exists between the ventricular system and the subarachnoid space because of an incomplete subarachnoid block |
|
|
What causes normal pressure hydrocephalus?
|
It's most commonly caused by subarachnoid hemorrhage or meningeal infection
|
|
|
What does normal pressure hydrocephalus look like?
|
Ventriculomegaly out of proportion to the degree of sulcal prominence
|
|
|
How can you tell NPH apart from atrophy?
|
-It can be very difficult
-MR CSF velocity and stroke volume calculations have been used -Radioisotope studies can be used -Remember that it’s not really a radiographic diagnosis |
|
|
What findings are seen on radioisotope cisternograms for NPH?
|
-Early entry of the radiopharmaceutical into the lateral ventricles
-Persistence of tracer within the ventricles at 24 and 48 hours -Considerable delay in the ascent to the parasagittal region |
|
|
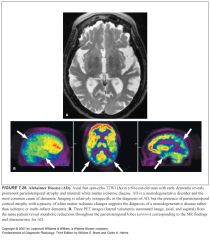
What is the most common neurodegenerative disease?
|
Alzheimer Disease
|
|
|
What two abnormal structures are seen on path in Alzheimer disease?
|
-Neuritic plaques—beta amyloid
-Neurofibrillary tangles—abnormal tau protein Both seem to interfere with normal neuronal functioning |
|
|
What does Alzheimer’s look like on imaging?
|
-Diffuse atrophy
-Predilection for the hippocampal formation, temporal lobes, and parietotemporal cortices |
|
|
How can you tell Alzheimer’s from age-related atrophy?
|
-Enlargement of the temporal hornas, suprasellar cisterns, and sylvian fissures
-Functional MR with regional cerebral blood flow calculations are being used to diagnosis and differentiate AS -PET may also play an important role |
|
|
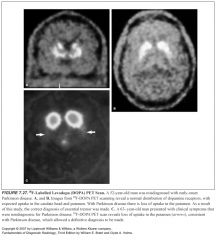
Explain the pathogenesis of Parkinson’s:
|
-There is dysfunction of the dopaminergic neuronal system, specifically the pars compacta of the substantia nigra
-This causes a deficiency of the neurotransmitter dopamine -When 80% of the dopaminergic neuronal cells die, pts have symptoms |
|
|
What percent of the dopaminergic neuronal cells have to die before Parkinson's pts develop symptoms?
|
When 80% of the dopaminergic neuronal cells die, pts have symptoms
|
|
|
What does the pars compacta look like on MR?
|
-High signal intensity band on T2
-Sandwiched between the pars reticularis anteriorly and the red nuclei posteriorly |
|
|
what does Parkinson’s look like?
|
-The high signal band of the pars compacta is lost
-This is only occasionally seen |
|
|
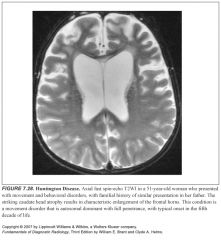
Explain the pathogenesis of Huntington disease:
|
-Autosomal dominant with complete penetrance
-Degeneration of the caudate -Movement disorder with dementia and emotional disturbances |
|
|
What does Huntingdon’s disease look like?
|
-Diffuse cortical atrophy
-The caudate nucleus and the putamen are most severely affected -Atrophy of the caudate nucleus results in characteristic enlargement of the frontal horns, which take on a heart-shaped configuration ("I heart Huntingdon's") |
|
|
What is Wilson disease also known as?
|
Hepatolenticular degeneration
|
|
|
Explain the pathogenesis of Wilson’s disease:
|
-Inborn error of metabolism
-Deficiency of ceruloplasmin (serum transport of copper) -Results in deposition of toxic levels of copper in various organs -Associted with hepatic cirrhosis and degenerative changes in the basal ganglia |
|
|
What physical exam finding is diagnostic of Wilson’s disease?
|
Kayser-Fleisher ring—intracorneal deposition of copper
|
|
|
What does Wilson’s disease look like on imaging?
|
-Diffuse atrophy
-Signal abnormalities involving the deep gray matter nuclei and deep white matter |
|
|
Aside from neurogenerative diseases, what can cause signal abnormalities in the basal ganglia?
|
Toxins
-Carbon monoxide -Methanol Infections -West Nile virus -Creutzfeldt-Jacob disease Benign calcifications -These can cause high signal on T1 -Related to the hydration layer effect where water molcules adjacent to calcifications have reduced relaxation times |
|
|
Why can basal ganglia calcifications have high signal on T1?
|
It's related to the hydration layer effect where water molcules adjacent to calcifications have reduced relaxation times
|
|
|
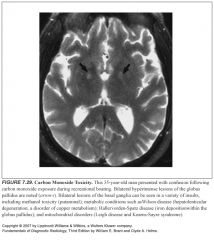
Where are signal abnormalities classically seen in carbon monoxide poisoning?
|
Globus pallidus
|
|
|
Where are signal abnormalities classically seen in methanol poisoning?
|
Putamen
|
|
|
1
|
|
|
2
|
|
|
3
|
|
|
4
|
|
|
5
|
|
|
6
|
|
|
7
|
|
|
8
|
|
|
9
|
|
|
10
|
|
|
11
|
|
|
12
|
|
|
13
|
|
|
14
|
|
|
15
|
|
|
16
|
|
|
17
|
|
|
18
|
|
|
19
|
|
|
20
|
|
|
21
|
|
|
22
|
|
|
23
|
|
|
24
|
|
|
25
|
|
|
26
|
|
|
27
|
|
|
28
|
|
|
29
|

