![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
109 Cards in this Set
- Front
- Back
|
What is signal based targeting? |
transport of protein from the cytoplasm to its ultimate location |
|
|
Is signal based targeting on a secretory or non secretory pathway? |
Non secretory |
|
|
What is vesicle based targeting? |
the transport from ER to its ultimate location using vesicles |
|
|
Is vesicle based targeting secretory or non secretory pathway? |
secretory |
|
|
What are some protein targeting challenges? |
1. Directing a protein to one specific area 2. Translocating a hydrophilic protein across a hydrophobic membrane |
|
|
What would the ulitimate destinations be in vesicle based targeting? |
1. Golgi 2. Nuclear envelope 3. Lysosomes/ endosomes 4. Vesicles 5. Vacuoles |
|
|
Where does the secretory pathway begin? |
In the ER |
|
|
What happens to proteins in the ER |
1. Glycosylation 2. Formation of disulfide bonds 3.Addition of lipids **Proteins can either stay in the ER, or be targeted elsewhere in the endomembrane system |
|
|
Proteins can either stay in the ER or....?
|
or can be targeted elsehwhere in the endomembrane system |
|
|
What is glycosylation ? |
Occurs to most proteins made in the ER |
|
|
Describe N-linked glyclosylation |
-Occurs in most proteins made in the ER( Soluble and transmembrane -Involves asn(N) and dolichol and transferase -Begins in Er and ends in Golgi ***mostly on lumenal side, thus chain always faces interior *** if this protein ends up in the PM,Oligo will face the ECM |
|
|
Where doe N-linked glycosylation begin and end? |
Begins in the ER , ends in Golgi |
|
|
What is dolichol? |
A lipid in the ER membrane --play a role in the co-translational modification of proteins known as N-glycosylation in the form of dolichol phosphate. |
|
|
What type of glycosyation occurs inthe cytosol or golgi? |
O-linked |
|
|
What occurs during O-linked glycosylation? |
Oligosaccharide is attached to an S or T residue at the -OH group |
|
|
During glycosylation if the protein ends up in the PM, _____1___ will face the ___2___ |
1. Oligo 2. ECM |
|
|
Where are disulfide bonds formed? |
Occurs mostly in ER lumed (rare in cytosol) |
|
|
How are disulfide bonds formed? |
1. Occurs mostly in ER lumen (rare in cytosol) 2. Protien disulfide isomerase(PDI) is oxidized by an ER protein Ero1-resulting in a disulfide bond in PDI's active site(removal of electrons) --Ero1 returns to its oxidized state by using O2 present in the ER lumen ---PDI then oxidizes the substrates cysteines |
|
|
What protein is used to form disulfide bonds? |
Ero1 |
|
|
How does Ero1 return to its oxidized state |
Using O2 present int he ER lumen |
|
|
What are the 3 steps of protein modifications in the ER |
1. Glycosylation 2. Formation of disulfide bonds 3. Addition of lipids |
|
|
What occurs when lipids are added to the protein modifications being made in the RER? |
1. Most occur in cytosol but some lipid modifications occur in the ER lumen --Signal =hydrophobic aa's --Involves GPI anchors (lipid + carbohydrate) --Enzyme cleaves C-term and attaches protein to GPI --Proteins will ultimately face extracellular space |
|
|
Where does the addition of lipids occur during protein modification |
Most occur in cytosol, some occur in ER lumen |
|
|
What are GPI anchors? |
-Lipid + carbohydrate -- glycolipid that can be attached to the C-terminus of a protein during posttranslational modification. --Glypiated (GPI-linked) proteins contain a signal sequence, thus directing them to the endoplasmic reticulum (ER). The protein is co-translationally inserted in the ER membrane via a translocon and is attached to the ER membrane by its hydrophobic C terminus; the majority of the protein extends into the ER lumen. The hydrophobic C-terminal sequence is then cleaved off and replaced by the GPI-anchor. As the protein processes through the secretory pathway, it is transferred via vesicles to the Golgi apparatus and finally to the plasma membrane where it remains attached to the a leaflet of the cell membrane. |
|
|
What is the point of glycosylation? |
To mature/ properly fold a protein ***if improper folding occurs, protein is ejected in cytosol and degraded |
|
|
What happens if improper folding occurs of a protein? |
if improper folding occurs, protein is ejected in cytosol and degraded
|
|
|
Steps of glycosylation during protein modification |
Signal (N-X-S/T) is recognized -->dolichol holds precursor oligosaccharide --> precursor is transferred toprotein, then trimmed
|
|
|
Where will proteins face after lipid addition during protein modification? |
extracellular space |
|
|
describe the secretory pathway |
1. Proteins with a ss are targeted to the ER 2. Vesicles bud off from ER and go to cis Golgi 3. Cisternal maturation in the GolgiFrom the trans Golgi network, proteins are loaded into different kinds of vesicles and trafficked to different destinations (primarily to the PM or lysosome) 4. Also: retrograde Golgi to ER transport and endocytosis that use similar mechanisms 5. In all cases, the proteins are NEVER in the cytosol |
|
|
what are vesicles? |
Small , spherical, membrane-bound chamberfor transporting cargo between compartments of the endomembrane system |
|
|
What is budding? (vesicles) |
Forming a transport vesicle |
|
|
What is fusion (vesicle) |
joinging a transport vesicle to an organell |
|
|
What is endocytosis? (vesicle) |
forming a vesicle from the PM
|
|
|
What is exocytosis (vesicle) |
fusing a vesicle to the PM |
|
|
What is a lumen? |
inner chamber -- Lumenof vesicles = topologically equivalent to OUTSIDE OF CELL --Cytosolicface of organelles and vesicles is equivalent to cytosolic face of plasmamembrane |
|
|
What are cargo receptors? |
Transmembrane proteins that cause a "clustering" of cargo |
|
|
What are adaptor proteins? |
(cytosolic)-recognize receptor clustering->recruits coat |
|
|
What are coat proteins |
cluster together and bend membane--> vesicle forms |
|
|
What are the function of destination markers for vesicle transport? |
mediates interaction with destination membrane |
|
|
What are the 3 main proteins used during vesicle formation? What are their functions? Where do they transport? |
1. Clathrin-coated (mainlyfor exocytosis and endocytosis) ---transportbetw. PM and endosomes, Golgi and PM, Golgi to endosome 2.COP1-coated (mainlyfor exocytosis or flow within EMS) ----transportbetw. Golgi and PM, Golgi cis and trans (both directions), Golgi to ER (ERretrieval) 3.COP2-coated (mainly for transport from ER tocis-side of Golgi)) |
|
|
Do all proteins first go to the ER?
|
No, proteins going to the mitochondria or chloroplasts or nucleus, as well as proteins that will remain in the cytosol, are made on free ribosomes that are not associated with the ER
|
|
|
What proteins are sent to the ER?
|
Proteins that are to be secreted out of the cell, as well as proteins bound for the plasma membrane, the Golgi apparatus and lysosomes plus those destined for the ER itself are some of the proteins sent to the ER. Those that are travelling beyond the ER move from the ER to the Golgi, and from there to the outside of the cell in secretory vesicles, if they are secreted proteins. If they are intended for the lysosome, the vesicles fuse with the lysosome to deliver them.
|
|
|
What vesicle making protein is mainly for exocytosis and endocytosis? |
Calthrin-coated |
|
|
What vesicle making protein transports between PM and endosomes, golgi and PM, golgi to endosomes |
Clathrin-coated |
|
|
What vesicle making protein is mainly for exocytosis or flow with EMS |
COP1-coated |
|
|
What vesicle making protein transports between golgi and pm, golgi cis and trans, golgi to ER |
COP1- coated |
|
|
What vesicle making protein is mainly for transport form ER to cis-side of golgi?
|
COP2-coated |
|
|
REVIEW SLIDE 16 |
CHAP 10-11 |
|
|
What are the 4 main steps to make COP2 veslicle? (ER to Golgi) |
1. Making vesicles requires G proteins (sar1/rab), V-SNAREs and coat proteins 2. Vesicle buds off: coat is shed exposing v-SNARE 3. Vesicles recognizes cis-Golgi: requires bindingbetween the small G-protein rab-GTP which attaches to vesicle and an effectoron the cis-golgi 4. Vesicle fuses with cis-Golgi; requires binding between V-SNARE and Golgi specific t-SNARE |
|
|
What G proteins are required for making a COP2 vesicle? |
sar1/rab |
|
|
What are V-SNARES? |
Special proteins called SNAREs are found on the surfaces of both transport vesicles (v-SNAREs) and of the target organelles (t-SNAREs). The docking of the v-SNARE with the t-SNAREs signals that the vesicle has reached its correct destination and can now fuse with the target organelle's membrane to deliver the contents. SNARE stands for soluble N-ethylmaleimide-sensitive factor attachment protein receptor).
|
|
|
What the the function of the cytosol |
contains many metabolic pathways; protein synthesis |
|
|
What the the function of the nucleus
|
contains main genome; DNA and RNA synthesis |
|
|
What the the function of the ER
|
synthesis of most lipids; synthesis of proteins for distribution to many organelles and to the plasma membrane |
|
|
What the the function of the golgi apparatus?
|
modification, sorting and packaging of proteins and lipids for either secretion or deliver to another organelle |
|
|
What the the function of the lysosomes?
|
intracellular degradation |
|
|
What the the function of the endosomes?
|
Sorting endocytosed material |
|
|
What the the function of the mitochondria
|
ATP synthesis by oxadative phosphorylation |
|
|
What the the function of the chloroplasts (plant cells)
|
ATP synthesis and carbon fixation by photosynthesis |
|
|
What the the function of the Peroxisomes
|
oxidation of toxic molecules |
|
|
Where are all proteins synthesized? |
By ribosomes in the cytosol |
|
|
How does a cell know where a particular protein should be sent?
|
Proteins have "address labels" or sorting signals that indicate which cellular compartment they are destined for. The sorting signals are part of the amino acid sequence of the proteins. Characteristic sorting signals are found on proteins that are sent to the nucleus, the mitochondria, etc.
|
|
|
How do proteins cross membrane barriers to get into the different compartments of the cell?
|
proteins going into the nucleus are transported through pores in the nuclear membranes with the help of nuclear transporter proteins. Nuclear proteins are transported in their folded state. However, proteins going into the ER enter the lumen (interior) of the ER through protein translocators in the ER membrane. In this case, the proteins must enter in an unfolded form, since the dimensions of the translocator will not allow a folded protein to pass through. A third mechanism encloses proteins inside a membrane vesicle, which travels to the target organelle and fuses with the membrane of the target.
|
|
|
What G protien is required for Coat assembly? |
sar1 |
|
|
How are coat protein in vesicle formation formed? |
Coat assembly requires the G-protein sar1, which when bound with GTP (sec12 is a GEF) has a conformation that embeds in membrane
|
|
|
What are the key transport steps in the formation of vesicles? |
1. Cargo selection 2. Vesicular budding 3. Vesicular targeting and fusion |
|
|
What are the 4 main steps to making a COPII vesicle? Where is it transporting from/to? |
Er to golgi 1. Making the vesicle reqires G-proteins (sar1/rab), v-SNARES and coat proteins 2. Vesicles buds off: coat is shed exposing v-SNARE 3. Vesicle recognizes cis-Golgi: requires binding between the small G-protein rab-GTP which attaches to vesicle and an effector on the cis-golgi 4. Vesicle fuses with cis-Golgi: requires binding between v-SNARE and golgi specific t-snare |
|
|
What G-protein is needed for a coat assembly |
sar1 |
|
|
What are the sole requirements for recognition specificity? |
Rabs and SNARES |
|
|
Give a brief outline of the secretory pathway. |
1. Proteins with a SS are targeted to the ER 2. Vesicles bud off from ER and go to cis Golgi 3. Cisternal maturation in the Golgi 4. From the trans golgi network, proteins are loaded into different kinds of vesicles and trafficked to different destinations (primarily to the PM or lysosome) 5. Also: retrograde golgi to ER transport and endocytosis that use similar mechanism ***THE PROTEINS ARE NEVER IN THE CYTOSOL |
|
|
Describe the golgi apparatus structrually |
1. 4-6 stacks of membrane-bound compartments called cisterna ---exhibits directeionaligy (enters on Cis side, leaves on trans( ---some proteins are retained by a golgi-retention signal or specialized glycosylations ---other proteins are sorted and move on |
|
|
What is the function of the golgi apparatus? |
1. Further trimming/ extending of N-linked glycsylations 2. Addition of O-linked glycosylation 3. Sort proteins to their ultimate destinations |
|
|
What are the two models of transport through the golgi? |
Vesicular shuttle and cisternae maturation model |
|
|
What is the function of dynamin? |
polymerizes around the neck and throught energy form GTP hydrolysis elongates, stretching the vesicle neck until it pinches off |
|
|
unlike COP1 and COPII vesicles clathrin/AP vesicles require________for the vesicles to pinch off |
dynamin |
|
|
What occurs during transport from the golgi? |
1. from the trans golgi network, vesicles bud off to go to various locations 2. These have different coat proteins (including clathrin and Adaptor protein(AP) complexes) depending on where they are going 3. Again requires recogniiton between protein sequences and coats for cargo loading and G-proteins for vesicle assembly and targeting |
|
|
How is materiel destined for exocytosis delivered to the PM? |
1. Constitutive secretory pathway 2. Regulated secretory pathway |
|
|
What is the constituative secretory pathway |
Pathway used for materials destined for exycytosis --no sorting signal required (default) --Constantly active, vesicles form golgi constantly fusing to the PM ---Only requires ER-import sequence (signal peptide) |
|
|
What signal is used in the constitutive secretory pathway? |
peptide |
|
|
What is the regulated secretory pathway? |
Pathway used for materials destined for exycytosis
----timed controlled ----vesicles form but do not fuse until signal is received(influx of Ca2+ ---- Common in neurotransmitter release, digestive enzymes |
|
|
What are lysosomes? |
Sites of intracellular digestion of macromolecules, organelles, etc.
----Contain 40+ hydrolases (lipases/proteases/nucleases/phosphatases/etc) -----These enzymes are first made as zymogens (inactive precursors) that become activated when cleaved in the lysosome Acidic pH (4.5-5.0) ---maintained by H+ pumps in membraneoptimal pH for hydrolases ---membrane protected by highly glycosylated proteins facing lumen |
|
|
What are the enzymes used in acid hydrolases (7). name at least 4 |
1. nucleases 2. proteases 3. glycosidases 4. lipases 5. phosphatases 6. sulfatases 7. phospholipases |
|
|
Describe the formation of a vesicle |
early endosome-->late endosome--> endolysosome--> lysosome 1. Early endosome forms when cell endocytoses material and forms a vesicle 2. Late endosome forms as vesicles from Golgi or other endosomes fuse and deliver material (enzymes) 3. Endosomes “mature” and proton pumps lower pH --> endolysosome and eventually -->lysosome |
|
|
Whats are zymogens |
Inactive substance that is converted into an enzyme when activated by another enzyme |
|
|
What is an endolysosome? |
The product of the fusion of an endosome and a lysosome during endocytosis.
|
|
|
What is a lysosome? |
an organelle in the cytoplasm of eukaryotic cells containing degradative enzymes enclosed in a membrane.
|
|
|
Can vesicles bring things into the cell?
|
Large molecules, and even bacterial cells or other large particles can be brought into cells, by a process called endocytosis. In endocytosis, vesicles effectively enclose an extracellular entity and bring it into the cell.
|
|
|
How does endocytosis occur
|
In endocytosis the plasma membrane surrounds the particle to be internalized and pinches off an internal vesicle (i.e., engulfs the particle).
|
|
|
How does receptor mediated endocytosis work?
|
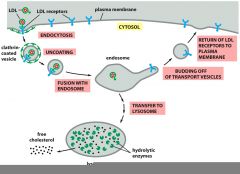
In receptor mediated endocytosis, the specific molecules to be brought into the cell first bind to protein receptors in special regions of the plasma membrane. These regions are called clathrin coated pits. Once the desired molecules have bound their receptors, the clathrin coated pits bud off into vesicles that carry the bound molecule into the interior of the cell.
|
|
|
What is an example of receptor mediated endocytosis?
|
The transport of cholesterol from the bloodstream into cells is a classic example of receptor mediated endocytosis. Cholesterol, packaged in particles called LDLs is bound by LDL receptors on the plasma membrane of the receiving cell, then internalized as vesicles containing the LDLs. The vesicles ultimately fuse with the lysosome where the LDLs are broken down to release free cholesterol, while the LDL receptors are recycled to the plasma membrane for re-use.
|
|
|
What are the functions of the Golgi apparatus
|
1. Processing and sorting of glycoproteins
2. Synthesis of glycolipids and sphingomyelin, a major component of membranes, especially in the brain. 3. In plant cells, Golgi complexes are involved in the synthesis of cell-wall polysaccharides like hemicellulose and pectin. |
|
|
Where do golgi complexes form? |
In the cytoplasm |
|
|
What do the Golgi complexes look like?
|
The Golgi complexes are composed of membrane-bounded sacs and vesicles that form flattened stacks in the cytoplasm. The side of the stack that is closest to the ER is called the cisface of the Golgi, while the side away from the ER is called the trans face of the Golg
|
|
|
What happens to proteins that come from the ER to the Golgi complex?
|
Proteins that arrive from the ER are taken up by the cis Golgi and passed through the stack. As they travel through the Golgi, they are further modified (recall that proteins have already been modified by the addition of various molecules such as sugars or lipids in the ER). These modifications help mark them for their final destinations. They are then passed from the trans Golgi into vesicles that travel to their targets and deliver the proteins to the desired region of the cell.
|
|
|
What kinds of modification do proteins undergo in the Golgi complex?
|
A major form of modification that proteins undergo in the Golgi complex is the alteration of glycoproteins (that is, proteins that have had sugars added to them in the ER). Many proteins that are glycosylated in the ER have an oligosaccharide added on to them. These proteins are modified by the clipping off of some of the sugars in the oligosaccharide and the addition of others in the Golgi complex
|
|
|
What is the significance of phosphorylation of the mannose sugars on lysosomal proteins?
|
The phosphorylation of mannoses serves to distinguish the lysosomal proteins from the other proteins. In glycoproteins going to the plasma membrane or which are going to be secreted, the first step in processing in the Golgi is removal of three mannose sugars attached to them.By contrast, in proteins destined for the lysosome, these mannose sugars have phosphate groups attached to them
|
|
|
What is special about the modification of proteins targeted to the lysosome?
|
Proteins that are to be sent to the lysosome receive special treatment in the Golgi complex. When these proteins enter the cis Golgi, they are phosphorylated on the specific mannose sugars attached to them. This results in the formation of mannose-6- phosphates on the lysosomal proteins.
|
|
|
How does the presence of mannose-6-phosphate target proteins to the lysosome?
|
The mannose-6-phosphate is recognized by a special receptor protein (M6PR) in the trans Golgi that then binds the lysosomal proteins and sends them to the lysosome.
|
|
|
What causes dissociation of M6P from M6PR, phosphate from mannose? What occurs after? |
1. Low pH
2. the enzyme is now retained in the lysosome |
|
|
What is retrograde transoprt form the cis-golgi to the ER involve? |
--returns SNAREs (in addition to misrouted proteins) back to the ER
--Uses similar mechanisms but have different coat proteins and G-proteins are called COP1 vesicles |
|
|
Durign endocytosis: Receptor medicated endocytosisuses ---1--- coated pits to form ---2-----containing specific things (again,requires energy)
|
1. clathrin 2. vesicles |
|
|
What does the hill activity assay measure? How can you tell the results? |
Measures the transfer ofelectrons from water to a DCPIP, which changes color based on its redox state
---Darkwhen oxidized; light when reduced |
|
|
What does the RT-PCR measure?How is it used? |
--Frequently used method to determine if a transcript is expressed
--From starting material to gel analysis (< 5 hrs) -----Extremely sensitive and can be used to quantify mRNA Approach: 1.purify mRNA 2.Add reverse transcriptase to make “cDNA” 3.Do PCR |
|
|
What is the northern blot? What is the issue with its funcitoning? |
1.One of the first techniques that enabled the detection of general or specific RNA transcripts in specific tissues – developed in 1977 2. don’t indicate where and for how long the protein functions |
|
|
What are some test that are used to examine gene expression |
1. Northern blot 2. In situ hybridization 3. Trangenic organisms |
|
|
What is in situ hybridzation? |
Use a labeled antisense RNA probe that can hybridize to endogenous transcripts (that are “sense”). Then use an enzyme-coupled antibody (i.e. anti-digoxigenin) to recognize the probe and produce a color.
---- Gives spatial and temporal resolution |
|
|
What is a transgenic organism? How is it used to examine gene expression |
--AnyDNA construct that is injected into cells at high constructions can randomlyintegrate into the genome (UNLIKEGENE TARGETING)
---Canbe used to overexpress genes under the control of specific promoter -----Tovisualize gene expression, have a marker (i.e lacZ, GFP) under the control of apromoter |
|
|
Expression of a gene/marker underthe control of a promoter is enhanced using the ________system
|
1. Gal4-UAS |
|
|
What is the Gal4-UAs system? |
----Gal4is a transcription factor
----UASis the DNA sequence that Gal4 recognizes Can also have separate lines thatexpress Gal4 that you then cross with other lines that have UAS |

