![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Alkenes |
Alkane/cycloalkane with c=c double bonds. P.p non polar.. soluble in non polar solvents ex gasoline... less dense than water |
|
|
|
Alkynes |
Alkane/cycloalkane with triple c-c bonds |
|
|
|
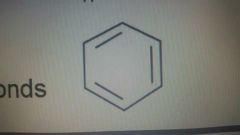
Aromatic compounds |
Special cycloalkane with double bonds |
|
|
|
Naming alkenes and alkanes |
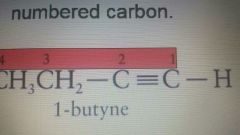
1. Name the longest chain that contains multiple Bond Use iupac root and the -ENE ending for double bond** -YNE for triple bond 2. Number them lowest number goes to the bond 3. Name any other branch and combine all |
|
|
|
1-butyne |
|
|
|
CH3-CH=CH-CH3 |
2-butene |
|
|
|
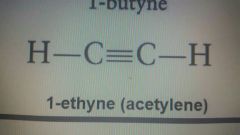
1-ethyne |
|
|
|
CH3-CH2-CH2-CH=CH-CH3 |
2-hexene |
|
|
|
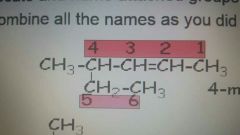
4-methyl-2-hexene |
|
|
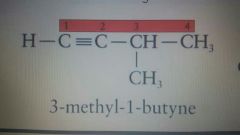
|
3-methyl-1-butyne |
|
|
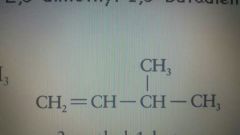
|
3-methyl-1-butene |
|
|
|
Stereoisomers |
Compounds with the same structural fomula but different spatial arrangements of atoms |
|
|
|
Geometric isomers |
A type of stereolsomer that gives "cis" and "Trans" orientation due to no free C-C rotation |
|
|
|
Cis |
Substituents on the same side |
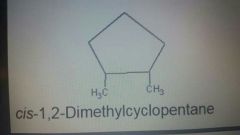
|
|
|
Trans |
Substituents on the opposite side |
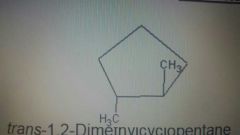
|
|
|
Geometric isomers for alkenes |
They exist when there are substituents attached to a c=c bond classified as cis or trans |
|
|
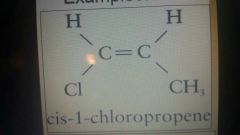
|
Cis cl and ch3 are on the same side |
|
|
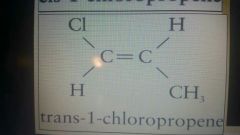
|
Trans. Cl and ch3 are opposite sides |
|
|
|
Aromatic compounds |
•special delocalized cycloalkanes • found in perfumes •most common type of aromatic compound is benzene |
|
|
|
Benzene |
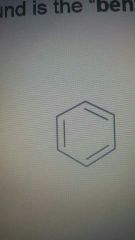
|
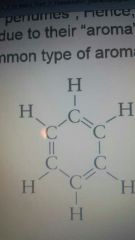
|
|
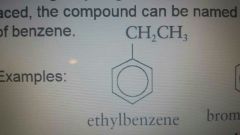
|
Ethylbenzene |
|
|
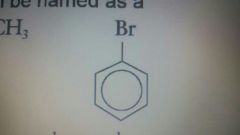
|
Bromobenzene |
|
|
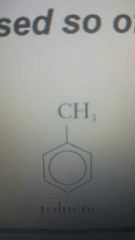
|
Toluene |
|
|
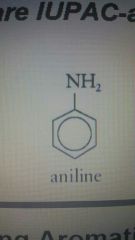
|
Aniline |
|
|
|
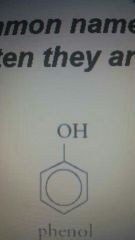
Phenol |
|
|
|
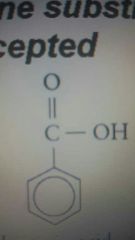
Benzoic acid |
|
|
|
Naming aromatic compounds with groups |
When two groups are attaches to the benzene ring their positions are designated (o,m,p) |
|
|
|
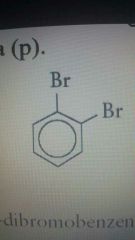
O position |
|
|
|
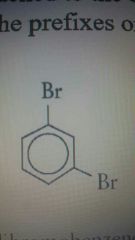
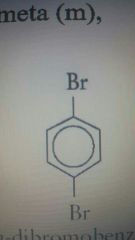
M position |
|
|
|
P position |
|
|
|
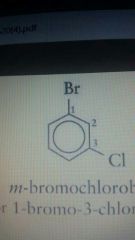
M-bromochlorobenzene Or 1-bromo-3-chlorobenzene |
|
|
|
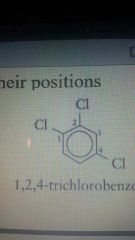
1,2,4-trichlorobenzene |
|
|
|
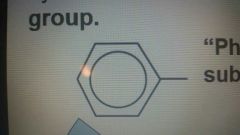
Phenyl group "substituent" |
|
|
|
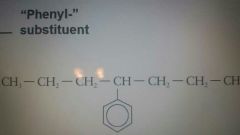
|
4-phenylheptane |
|
|
|
P.p. aromatic compounds |
•non polar (insoluble in water//soluble in gasoline) •lipophilic |
|

