![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
G₁ Phase |
First component of interphase
Major growth phase
First major checkpoint in the cell cycle occurs at the G1/S border - cell must determine if it has the necessary energy and nutrients to begin DNA synthesis and enter cell cycle - cell must determine if the environment is right to divide - cell must synthesize proteins and buildup redox potential |
|
|
S Phase |
Second component of interphase
DNA replication occurs in this phase in preparation for division later on in the cell cycle
|
|
|
G₂ Phase |
Third and final component of interphase
Second growth period and second checkpoint of cell cycle - cell must check to make sure there were no issues with DNA replication before committing to cell division - cell grows to create enough organelles and cytoplasm for division into two daughter cells |
|
|
M Phase |
Cell division occurs during this stage
Once a cell commits to M phase it must complete the process; there is no going back
Third checkpoint occurs before the transition from metaphase to anaphase - cell checks to make sure all chromosomes are attached to their spindles - if so, anaphase and cytokinesis are initiated |
|
|
G₀ Phase |
Resting phase partially removed from regular cell cycle
Cells maintain normal cellular function w/o replication
Often occurs in senescent cells and terminally differentiated cells (e.g. cardiomyocytes, neurons) |
|
|
Cell Cycle Control System |
A series of biochemical switches induce and inhibit the stages of the cell cycle
Major component = cyclin-dependent kinases
Biochemical processes involved - protein phosphorylation - protein-protein interactions - protein degradation - telomeres |
|
|
Cyclin-Dependent Kinases (Cdks) |
Phophorylate proteins to drive the progession of the cell cycle
Only active when bound to a cyclin - causes binding loop in Cdk to become more accessible
Fully activated when binding loop is phosphorylated by a Cdk-activating kinase
Specificity for activity is cyclin dependent
|
|
|
G₁-CDK Cyclin-Cdk Complex |
Cyclin D and Cdks 4 & 6 |
|
|
G₁/S-CDK Cylcin-Cdk Complex |
Cyclin E and Cdk 2 |
|
|
S-Cdk Cylcin-Cdk Complex |
Cyclin A and Cdks 2 & 1 |
|
|
M-Cdk Cyclin-Cdk Complex |
Cyclin B and Cdk 1 |
|
|
Time Dependent Control of Cell Cycle Using Cyclins |
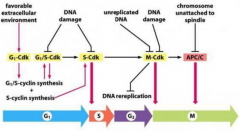
each progressive Cdk-cyclin pair acting in the cell will influence expression levels of other Cdk-cyclin pairs by up-regulation or down-regulation |
|
|
Inhibition of CDK activity [Diphosphorylation] |
Kinases can also inhibit Cyclin-Cdk complexes by phosphorylating them on a different site - Wee1 phophorylates the complex to inhibit it - Cdc25 phosphatase dephosphorylates it |
|
|
Inhibition of CDK activity [CKIs] |
Cyclin dependent kinase inhibitory proteins (CKIs) for a heterotrimeric complex with the active Cyclin-Cdk complex
Example: p27 binds and obscures the active site on the active Cdk2/4-cyclin complex |
|
|
Inhibition of CDK activity [INK4] |
INK4 proteins A and B can bind to Cdk 4 and 6 and sequester them away from Cyclin D thus preventing their activation |
|
|
Cell Cycle Regulation via Proteolysis |
Controlled by multi-protein ubiquitin ligases
Can stop or induce a phase of the cell cycle depending on what type of protein it degrades |
|
|
Cell Cycle Regulation via Proteolysis [APC/C] |
Stops phase of cell cycle by degrading cyclins
Specifically targets Cyclin B for degradation at the end of M phase of cell cycle
Inactive APC/C binds with Cdc20 to become active
Adds poly-ubiquitin tail to Cyclin B targeting it for degradation by proteosomes |
|
|
Cell Cycle Regulation via Proteolysis [SCF] |
Induces phase of cell cycle by degrading CKIs
CKIs are first phosphorylated by a kinase (ex: PLK 3)
Then can then bind to the F-box protein in the activated SCF complex and be poly-ubiquitinated and targeted for proteosomal degredation |
|
|
Replicative Cell Senescence |
Cells can only divide a limited number of times before being undergoing permanent cell cycle arrest -typically 50-70 times
This occurs because our telomeres are shortened after each successive cell division - when they reach a critical length the cell is forced to stop dividing |
|
|
Control of S Phase by S-Cdks |
Pre-Replication complex, containg Cdc6 and Cdt1, bind to DNA and Helicase; prevent helicase from proceeding down DNA
Active S-Cdk phophorylates Cdc6, priming it for polyubiquitination and degradation - this also causes it to dissociate from the DNA and Cdt1
Cdt1 can then be sequestered by geminin
The pre-initiation complex can now bind to the DNA where it is phosphorylated by S-Cdk
This causes the pre-IC to recruit polymerase and initiates replication |
|
|
Control of M Phase by M-Cdks |
Cdk1 and Cyclin B bind, but the complex in inactive
It is simultaneously diphosphorylated by both CAK and WEE1 which causes the complex to remain inactive
When the cell is ready to enter mitosis, Cdc25 phosphatase removes the inhibitory phosphate that Wee1 added thus activating the M-Cdk complex
The M-Cdk complex then up-regulates its own activity by 1. Phosphorylating and inactivating Wee1 2. Phosphorylating more Cdc25
This is why once you commit to M phase there is NO GOING BACK! |
|
|
APC/C M-Cdk Dependent Initiation of Anaphase |
M-Cdk complex serves to further activate APC/C
M-Cdk also activates ERK which phosphorylates Securin
Activated APC/C polyubiquitinates the phosphorylated Securin which is inhibiting the action of Seperase
Once the Securin is degraded, Seperase goes on to seperate chromosomes during anaphase |
|
|
Control of Cytokinesis by GTP-dependent Formation of Contractile Ring |
Actin/Myosin rings form around cell during cytokinesis
RhoA activation causes activation of these filaments in the ring which leads to contraction and formation of the cleavage furrow
Regulated by Cdk1 and cyclin B because this occurs in M phase |
|
|
Induction of G1 Cell Cycle by Growth Factors |
GF binds to RTK which autophosphorylates itself and recruites PI3K
PI3K phosphorylates PI(4,5)P₂ into PI(3,4,5)P₃
PIP₃ activates AKT(Protein Kinase B) which phosphorylates MTOR
MTOR activates the TOR complex which initiates translation and protein synthesis by activating S6K and inhibiting 4E-BP |
|
|
Mitogen Activated Cell Signaling and S Phase Entry |
Mitogen binds to receptor which leads to phosphorylation and activation of Ras
Ras phosphorylates MEK which phosphorylates ERK
ERK phosphorylates the transcription factor Myc
Myc facilitates transcription of G1 cyclin (D)
Cdks 4 and 6 phosphorylate Rb causing it to dissociate from E2F
|
|
|
Excessive mitogen-activated signaling can induce apoptosis or arrest
|
Myc facilitates trascription and translation of protein Arf
Arf sequesters Mdm which usually targets p53 for degradation
Non sequestered, active p53 can 1. Arrest the cell cycle in favorable environment 2. Initiate Apoptosis in unfavorable environment |
|
|
DNA Damage Halts the Cell Cycle |
Cell damage activates ATM/ATR kinases
ATM/ATR phosphorylate Chk1/Chk2 which phosporylate p53
Phosphorylated p53 induces expression of p21, a CKI
p21 binds to G₁/S and S-CdkC |
|
|
Cdks and Cancer |
Normal Cell: - Highly active CKIs - Normal levels of Cdk-cyclin pairs
Cancer Cell: - CKI expression remains relatively the same - Highly overactive Cdk-Cyclin expression |

