![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
171 Cards in this Set
- Front
- Back
|
What is the composition of blood? |
Plasma - 2/3 - water, salts, enzymes, globulins, clotting factors (cryoprecipitate - a source of fibrinogen) White blood cells Red blood cells - 1/3 Platelets
|
|
|
What do haemoglobin levels tell us? |
The concentration of red blood cells Low - anaemia High - dehydration |
|
|
Describe the terms for too many platelets and too little. |
Too little - thrombocytopenia Too many - thrombocytosis |
|
|
Give the cut off values of haematocrit and haemoglobin for pregnant and non pregnant women which are used to define anaemia. |
NON-PREGNANT: Haematocrit (volume of red blood cells) - 36% Haemoglobin - 12g/dL
PREGNANT: Haematocrit - 33% Haemoglobin - 11g/dL |
|
|
What happens to plasma volume and red cell mass during pregnancy? |
They increase. Plasma - 2600ml Red cell mass increases by 17% in normal pregnancy. Erythropoietin rises int eh first trimester. But there is a plateau in the last 8 weeks. Non-pregnant levels reached again by 3 weeks after delivery. |
|
|
How is excess iron stored in the body? |
In the bone marrow as ferritin. In the liver as haemosiderin.
|
|
|
Give the daily iron requirements for men, menstruating females, and pregnant women. |
Male - 1mg MF - 2mg PW - 4-7mg |
|
|
How do you assess low haemoglobin outside pregnancy? |
1. Is the Hb below the normal range? 2. Check the mean cell volume (normal range = 80-100fl) <80 - iron deficiency or haemoglobinopathy >100 - B12/folate deficiency or haematological problem
|
|
|
When would you diagnose iron deficiency during pregnancy? |
Haemoglobin less than 105g/l but consider if less than 115 Mean cell volume is less than 80 Serum ferritin is less than 15mcg/l |
|
|
How do you manage iron deficiency anaemia? |
They need 120-140mg elemental iron each day (not all is absorbed). It is better to take oral tablets and it is better to take tablets with at lease 60mg of elemental iron so you don't have to take so many. Eg. Ferrous fumarate, sulphate and sulphate (dried) But you can also use ferrous gluconate and succinate |
|
|
What are the folate requirements during pregnancy and which women need folate the most? |
300-400ug/day (normally 100) Need folate in twin pregnancies and hyperemesis gravidarum (bad morning sickness) |
|
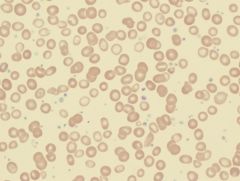
Define the condition the sample is showing. |
Thrombocytosis. Platelets >500x10-9/l |
|
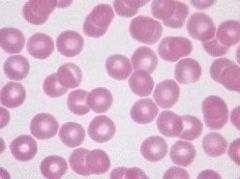
Define the condition the sample is showing. |
Thrombocytopenia. Platelets <150x10-9/l |
|
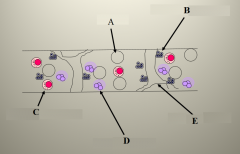
Define labels A-E on the bone marrow. |
A - Fat cells B - Erythroid precursors C - Myeloid precursors D - Megakaryocytes E - Bony trabeculae |
|
|
List the differences between healthy marrow and failing bone marrow. |
Failing bone marrow just has fat cells and bony trabeculae. It is missing the erythroid precursors, myeloid precursors and megakaryocytes that healthy bone marrow has. |
|
|
What happens when bone marrow is infiltrated with malignant cells? |
Anaemia and low white cell count (leucopenia) - there is no space to make other cells. Thrombocytopenia - there is increased consumption of platelets outside the marrow, this causes increased numbers of megakaryocytes within the bone marrow. |
|
|
What are the differential diagnoses for thrombocytopenia in pregnancy? |
Pregnancy induced hypertension HELLP - haemolysis, elevated liver enzymes, low platelets Disseminated intravascular coagulation - HELLP, massive haemorrhage, amniotic fluid embolism |
|
|
Give the differential diagnoses for thrombocytopenia for non-pregnancy related cases. |
Immune thrombocytopenia - autoimmune condition Familial thrombocytopenia Drugs Pseudothrombocytopenia (Platelet clumping in EDTA) Bone marrow infiltration Hypersplenism - a third of platelets made will go to the spleen |
|
|
Describe the pathogenesis of venous thromboembolism in pregnancy. |
INCREASED VENOUS STASIS - increased capacitance and venous distensibilty, compression of IVS and left iliac vein, 50% reduction in venous flow ENDOTHELIAL INJURY - at time of delivery HYPERCOAGUABLE STATE - increased procoagulants, reduced anti-coagulants. |
|
|
Give the clinical presentations of PE and DVT. |
PE - dyspnea, pleuritic chest pain, cough, haemoptysis, syncope
DVT- leg swelling, pain, tenderness |
|
|
How do you diagnose PE and DVT? And what is the treatment? |
DVT - Doppler USS PE - CT scan of pulmonary vessels
Treatment - low molecular weight heparin |
|
|
Give the definitions of: A Blood product A Blood component A plasma derivative |
Blood product = therapeutic substance prepared from human blood Blood component = Made from the major constituent parts (eg. red cells, platelets, white blood cells, plasma) Plasma derivative = plasma proteins prepared from pooled human plasma (eg. albumin, coagulation factors)
|
|
|
Which processes does donated blood undergo before it can be given to a patient? |
1. Tested by blood-borne diseases 2. The white blood cells are removed 3. It is separated into red cells, pooled platelets and fresh frozen plasma 4. The compatibility of the red blood cells is checked |
|
|
Describe the special requirements regarding donated red blood cells. |
Irradiation - this destroys the ability to replicate any residual WBCs, particularly lymphocytes - it stops transfusion associated graft versus host disease (residual lymphocytes graft in person and destroy the cells - fatal) Required for bone marrow transplant patients and from transfusion from family CMV negative blood - Cytomegalovirus infection can cause serious morbidity in immunocompromised CMV negative patients. The risk is reduced by use of CMV - antibody negative blood components |
|
|
Give the indications for use for fresh frozen plasma. |
To replace clotting factor deficiencies when a suitable specific or combined factor concentrate is not available. To reverse warfarin If the patient has had a massive transfusion Liver disease
|
|
|
Give the different types of fresh frozen plasma. |
Standard FFP - 1 uk male donor, ABO compatible, variable coagulation factors - need high dose to get coagulation factors up Methylene blue plasma - 1 US donor, for children Solvent-detergent plasma - from thousands of donors, very little fibrinogen - safe Cryoprecipitate - the richest source of fibrinogen
|
|
|
Describe the ABO system in terms of genes. |
The A and B genes encode for enzymes (transferases) which transfer the appropriate sugars to substance H, resulting in A and B antigens. The O gene is an amorph (has no phenotypic affect) and does not code for any transferase |
|
|
Describe the ABO system in terms of antibodies. |
In the serum of individuals are naturally occurring antibodies to the antigens which are absent on their red cells. These naturally occurring antibodies are primarily IgM and would readily destroy incompatible red blood cells. O has no antigens present which is why it is a universal donor. |
|
|
For each blood type, give the antibodies present in their serum and the blood they can receive from others. |
A - anti-B - A,O B - anti-A - B,O AB - none - AB, A, B, O O - anti-B anti-A - O |
|
|
Briefly explain the Rhesus system. |
Rhesus positivity or negativity usually refers to the presence or absence of the D antigen. Only 15% of the Caucasian population are RhD-negative. If RhD-negative individuals are exposed to RhD-positive red cells, a significant proportion would make anti-D antibodies in response. |
|
|
What is the significance of the Rhesus system to pregnant women? |
Immune ant-D antibodies are IgG and so can cross the placenta. If an RhD-negative pregnant woman is carrying an RhD positive foetus, the anti-D can cross the placenta and destroy the foetus' red cells. |
|
|
What can happen to an RhD-positive foetus if exposed to anti-D? How can this be prevented? |
The foetal red cells are destroyed leading to haemolytic disease of the newborn (HDN- this causes jaundice, hepatosplenomegaly, hypoglycaemia, bilirubin encephalopathy, pericardial/pleural effusion, it can even result in stillbirth) Need to prevent RhD negative women being sensitised to RhD-positive blood. |
|
|
When does sensitisation to RhD positive blood commonly occur? |
When a RhD-negative mother gives birth to a RhD- positive baby. There is bleeding from the placenta and blood can mix. The first child is therefore unaffected, it any RhD-postiive babies conceived after that are at risk of moderate to severe HDN. |
|
|
How do we prevent sensitisation of RhD-negative women during pregnancy? |
Anti-D if given after each potentially sensitising event during the pregnancyand at 28 weeks and 34 weeks and within 72hours of childbirth (if the baby is RhD-positive. The administered anti-D would destroy any RhD- positive foetal cells in the maternal circulation before the mother has had the opportunity to make anti-D in response. |
|
|
What is transfusion associated circulatory overload? |
Patient is overloaded with blood. Occurs when the transfusion is not necessary, or the volume is too large, or the transfusion is done too rapidly, if the patient is elderly or has chronic anaemia. Symptoms include - dyspnea, orthopnea, peripheral oedema and rapid increase of blood pressure. |
|
|
What is transfusion related acute lung injury? |
Acute lung injury related to blood transfusion. It is caused by the presence of leukocytes antibodies in transfused plasma. Leukoagglutination and pooling of granulocytes may occur in the lungs with release of the leukocyte's granules and so injury to cellular membranes, endothelial surfaces and lung parenchyma. This causes pulmonary oedema. It presents with sudden dyspnea, severe hypoxemia, hypotension, and fever that develop within 6 hours of the transfusion taking place. It usually resolves within 48-96 hours with supportive care. |
|
|
What is programming? |
An insult or stimulus which occurs at a critical period in development and which has lasting or lifelong consequences. |
|
|
Why is a low birth weight linked to diabetes? |
The fetus has become metabolically thrifty in adverse nutritional circumstances - this thrift among other things results in impaired growth of beta cells in the pancreas. This is due to villi in the trophoblasts getting thickened due to inflammation which can impar transport of nutrients (glucose) down the concentration gradient from mother to fetus through syncytial knots - impaired glucose tolerance in later life. |
|
|
What is the brain sparing effect? |
A physiological mechanism used by a foetus to increase blood supply to it's brain at the expense of other organs. The three most important organs to a foetus are the brain, heart and the adrenals. Evidence for this effect includes pancreas weight being lighter with less beta cells, kidney weight and nephron number decreased, fatty liver, small abdominal organs, resistance to blood flow in superior mesenteris artery. But not just the blood flow is altered - cell signalling is changed, hormone inducers, nutrients and oxygen levels are altered. |
|
|
When a baby is born, physiologically, what occurs after the umbilical cord it cut? How does this change if the mother has diabetes? |
The baby's nutrient supply is cut off and blood glucose starts to fall. Insulin levels also fall. Glucagon levels increase. Glycolysis occurs in the liver. Ketogenesis occurs - fat breaks down. Gluconeogenesis starts in the liver. But if the mother has diabetes she and the baby both have high blood sugar and when the glucose drops, insulin levels are high and so glycogenolysis can't occur. |
|
|
Describe the effects of DHA deficiency. |
DHA is an omega-3 fatty acid that is a primary structural component of the brain, skin, retina, sperm and testicles. DHA deficiency is associated with cognitive decline. It causes impaired gene expression for regulation of neurogenesis, decreased neurotransmission and connectivity, irreversible deficits in serotonin and dopamine release and can cause fine motor deficits. It is also associated with decreased gestation length. |
|
|
What are the risks of excessive trans-fats consumption in pregnancy? |
They directly cross the placenta and assimilate in foetal tissues. They are associated with growth restriction and the development of cardiovascular disease. |
|
|
Give the health benefits of breastfeeding. |
It improves glucose intolerance, hypertension, reduces the chances of obesity, it reduces the increase in adiposity levels associated with exposure to diabetes in utero, it lowers cholesterol after childhood, boosts the childs immune system, and mum burns calories. |
|
|
What is amniotic band syndrome? |
Sometimes tears form in the amniotic membrane and a band may encircle part of the fetus (eg, limb or digit). When the tear tries to repair the amnion constricts around the interposing tissues causing amputations or other abnormalities. |
|
|
Where does the cardiovascular system originate from? |
The mesodermal germ layer. |
|
|
Describe the 2 different mechanisms that are involved in the formation of the cardiovascular system. |
Vasculogenesis - development of blood vessels occurs from endothelial cells differentiating locally in position. (genetic)
Angiogenesis - formation of new blood vessels from sprouting of new capillaries from pre-existing vessels. |
|
|
Describe the early stages of vasculogenesis. |
The blood vessel development starts in the yolk sac and the trophoblast layer. It starts (around the same time as somitogenesis) with formation of mesodermal aggregates called blood islands, composed of haemangioblasts in the extraembryonic tissues. Endodermal-mesodermal interactions are necessary for blood island formation and the fibroblast and transforming growth factors play a role in the mesodermal induction. |
|
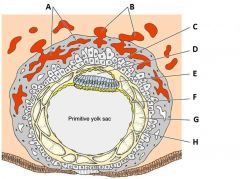
Define the labels A - H on the diagram of a 12 day old placenta. |
A - Trophoblastic lacunae B - Maternal blood vessels C - Amniotic ectoderm D - Amniotic cavity E - Embryonic epiblast F - Hypoblast G - Extraembryonic coelom H - Extraembryonic mesoderm |
|
|
Give the functions of the decidua (endometrium, lining of uterus). |
A source of nourishment for the early embryo. Limits the invasiveness of the trophoblasts. Helps form the maternal component of the placenta (ie. partially eroded to form the intervillous space); remainder forms the decidua basalis Forms a cleavage plane - the placenta in uterus separate during labour |
|
|
What is the function of syncitiotrophoblasts? |
They prevent clot formation in the maternal blood within the intervillous space of the placenta. |
|

Define the labels A - I on the diagram of the placenta at the end of the 3rd week of pregnancy. |
A - Tertiary villi B - Intervillous spaces C - Syncytium D - Outer cytotrophoblast shell E - Connecting stalk F - Amniotic cavity G - Yolk sac H - Chorionic plate I - Chorionic cavity |
|
|
Give the description of the primary, secondary and tertiary villi. |
Primary - Core of cytotrophoblasts covered by a layer of syncytiotrophoblasts Secondary - Same as primary but a core of mesoderm Tertiary - Same as secondary but with less syncitiotrophoblasts and with the mesoderm showing capillaries and venules |
|
|
What happens histologically with the vascular at the end of the third week? |
Maternal vessels penetrate the cytotrophoblastic shell to enter the intervillous spaces, which surround the villi. The capillaries in the villi are in contact with vessels in the chorionic plate and in the connecting stalk, which in turn connect to the intraembryonic vessels. |
|
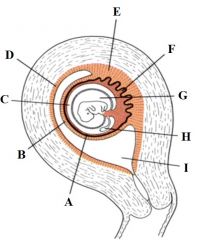
Define the labels A - I from the diagram of a uterus at the 2nd month of pregnancy. |
A - Chorion laeve B - Decidua capsilaris C - Chorionic cavity D - Decidua parietalis E - Decidua basalis F - Chorion frondosum G - Amniotic cavity H - Yolk sac I - Uterine cavity
|
|
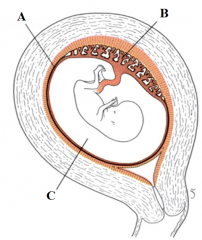
Define the labels A - C from the diagram of a uterus at the end of the 3rd month of pregnancy. |
A - Fused decidua parietalis, chorion laeve and amnion B - placenta C - Amniotic cavity |
|
|
Describe the foetal portion of the placenta. |
It is formed of the chorion frondosum or "bushy" chorion formed by the growth of villi on the embryonic pole. |
|
|
Describe the maternal portion of the placenta. |
Formed of the decidua basalis. On the foetal side, the placenta is bordered by the chorionic plate and on it's maternal side, by the desidual basalis. |
|
|
Give the unusual features of the placenta. |
It has dual origin from fetal and maternal tissue. It has exposure of maternal blood to non-maternal trophoblast tissue. It has simultaneous execution of diverse tasks - gas exchange, nutrient transport, excretion, hormone and protein synthesis Limited life span - disposable organ |
|
|
Give the layers of the fetomaternal barrier (placenta) from maternal side down to the foetal side. |
Syncytiotrophoblasts Cytotrophoblasts Basal lamina under the cytotrophoblasts Basal lamina of the endothelium of foetal capillary Endothelium of venous capillary |
|
|
What do blood islands form and where? |
Blood islands spontaneously differentiate and form endothelial cell lined vesicles that contain immature blood cells (haemangioblasts).
|
|
|
What are angioblasts? |
Differentiated endothelial cells in the embryo that DO NOT surround blood cells. |
|
|
What are the next stages in vasculogenesis once the blood islands have formed? |
The blood islands fuse and give rise to a primary vascular (capillary) plexus. This step requires intracellular signalling from the embryo. |
|
|
What is the common progenitor for hematopoietic and vascular endothelial cells. |
Haemangioblasts. |
|
|
What are the main differences between vasculogenesis and angiogenesis? |
Vasculogenesis is restricted to embryonic development whereas angiogenesis can occur during the entire lifespan of the organism. Angiogenesis - more stimulation from environment. |
|
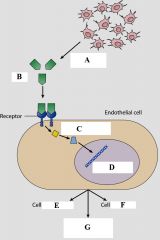
Define the labels A - G for the process of angiogenesis |
A - Embryonic tissue B - Vascular endothelial growth factor (VEGF) C - Signal transduction proteins D - Genes activated in nucleus E - Proliferation F - Migration G - Secretion of matrix metalloproteinases (MMPs) |
|
|
What are the first signs of heart formation? |
Formation of clusters of endocardial cells (angioblasts) and cardiac myoblasts from the mesoderm of the heart forming regions (starts 17-18 days).
|
|
|
What are the next steps in the formation of the heart? |
The clusters coalesce to form the heart tube. Alongside, other clusters of cells form the dorsal aortae (by vasculogenesis). With cephalo-caudal folding, the heart tube and the pericardial cavity are pushed towards the thoracic region (20-22 days). The heart tube shows 5 dilatations along the length of the tube. |
|
|
What are the 5 dilatations along the length of the heart tube? |
Truncus arteriosus, Bulbus cordis, Primitive ventricle Primitive atrium, Sinus venosus |
|
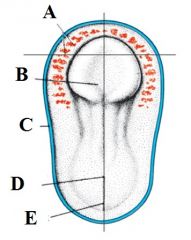
Define the labels A - E for the late presomite stage embryo (day 18) |
A - Myoblasts and blood islands B - Neural palte C - Cut edge of amnion D - Primitive node E - primitive streak |
|
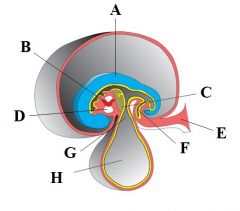
Define the labels A - H on the diagram depicting cephalo-caudal folding |
A - Neural plate B - Heart tube C - Septum transversum D - Pericardial cavity E - Connecting stalk F - Allantois G - Vitelline duct H - Yolk sac |
|
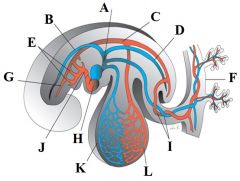
Define the labels A - L on the diagram of the cardiovascular system at 4 weeks. |
A - Common cardinal vein B - Anterior cardinal vein C - Dorsal aorta D - Posterior cardinal vein E - Aortic arches II and III F - Placenta G - Internal carotid artery H - Heart I - Umbilical vein and artery J - Aortic sac K - Vitelline vein L - Vitelline artery |
|
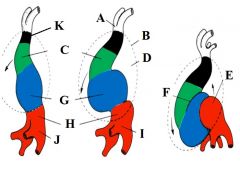
Define the labels A - K on the diagram depicting dextral looping. |
A - Aortic roots B - Pericardium C - Bulbis cordis D - Pericardial cavity E - Left atrium F - Bulboventricular sulcus G - Ventricle H - Atrium I - Sinus venosus J - Sinus venosus K - Truncus arteriousus |
|
|
What is dextral looping? |
The primitive tube bends to the right. This corrects the blood flow from entering the left ventricle so that it enters the right ventricle (as it does in the adult heart). The venus inflow goes through the sinus venosus then arterial out through the truncus arteriousus |
|
|
When does the heart divide into a typical 4 chambered structure? |
during the 4th to 7th weeks. Septum formation arises in part from the atrioventricular cushions and the conotruncal swellings. |
|
|
How does the conducting system of the heart develop? |
At week 5 cardiomyocytes of the sinus venosus undergo spontaneous depolarisations at a faster rate than cells in other regions. As looping occurs, the SV is incorporated into the right atrium and these fast-rate depolarising myocytes become the sinoatrial node. The atrioventricular node and bundle of his are derived from cells of the SV and cells from the atrioventricular canal. |
|
|
Describe fetal circulation from placental blood entering the system to blood reaching the left ventricle. |
Placental blood (80% oxygen saturated) returns to the fetus via the umbilical vein. The blood flows through the ductus venosus (through the liver) into the inferior vena cava. Here the blood mixes with deoxygenated blood and enters the right atrium, passes through the foramen ovale and into the left atrium. In the left atrium it mixes with a small amount of desaturated blood from the lungs and blood enters the LV and ascending aorta. |
|
|
Describe fetal circulation from blood leaving the ascending aorta to the blood flow back to the placenta. |
From the ascending aorta the blood flows through the carotid and coronary arteries which are the first branches of the AA. So the heart and brain are supplied with well oxygenated blood. Desaturated blood flows from the SVC via the right ventricle into the pulmonary trunk (where resistance is high) and thus most of this blood passes directly through the ductus arteriosus into the descending aorta (mixes with blood from proximal aorta). From here it flows to the placenta via the 2 umbilical arteries (58% O2). |
|
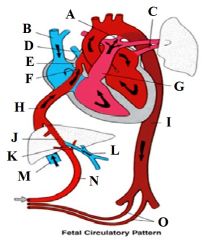
Define the labels A - O on the diagram depicting the fetal circulatory pattern. |
A - Ductus arteriosus B - SVC C - Pulmonary vein D - Pulmonary vein E - Crista dividens F - Foramen ovale G - Pulmonary artery H - Inferior vena cava I - Descending aorta J - Ductus venosus K - Sphincter in ductus venosus L - Portal vein M - Inferior vena cava N - Umbilical vein O - Umbilical arteries |
|
|
What are the 4 main circulatory changes at birth? |
Closure of ductus arteriosus Closure of the umbilical arteries Closure of the foramen ovale Closure of umbilical vein and ductus venosus |
|
|
Describe the closure of the ductus arteriosus. |
Its closed by muscle wall contraction (occurs almost immediately after birth). It's caused by bradykinin released from lungs at first inflation. It takes 1-3 months for total obliteration, forming the ligamentum arteriosum. |
|
|
Describe the closure of the umbilical arteries. |
Its closed by wall contraction (caused by thermal/mechanical stimuli and change in O2 tension). It happens a few minutes after birth. It takes 2-3 months for total lumen obliteration. |
|
|
Describe the closure of the foramen ovale. |
The closure results from pressure increase in left atrium and decrease on the right side. The first breath is important. Crying by the baby creates a shunt right to left. |
|
|
Describe the closure of the umbilical vein and ductus venosus. |
The closures occur shortly after the closure of the umbilical arteries. Thus blood may enter the newborn for some time after birth. Once obliterated they form the ligamentum teres hepatis and the ligamentum venosum. |
|
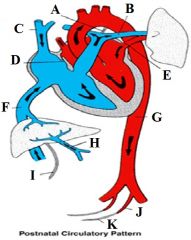
Define the labels A - K on the diagram depicting the postnatal circulatory system. |
A - Ligamentum arteriosum B - Pulmonary artery C - SVC D - Closed foramen ovale E - Pulmonary vein F - IVC G - Descending aorta H - Portal vein I - Ligamentum teres hepatis J - Superior vesical artery K - Medical umbilical ligament |
|
|
Name some cardiac teratogens. |
Rubella, Thalidomide (morning sickness drug) Vitamin A (retinoic acid) Alcohol |
|
|
What periods in cardiovascular development can teratogens cause major and minor birth defects? |
Major birth defects - 2.5 - 5.5 weeks Minor birth defects - 5.6 - 8 weeks |
|
|
Give 5 common congenital heart defects. |
Ventricular septal defect. Tetralogy of fallot (pulmonary stenosis - pulmonary artery, ventricular septal defect, over-riding aorta - aorta is next to the ventricular septal defect, allowing oxygen poor blood to flow through it and a thick right ventricle) Patent ductus arteriosus - failure of the ductus arteriosus to close after birth Transposition of great vessels Valvular stenosis |
|
|
Describe the upper uterine segment. |
This portion of the uterus consists of the fundus and that part of the uterus lying above the reflection of the vesico-uterine fold of peritoneum. During preganancy it undergoes the greatest degree of myometrial hyperplasia and hypertrophy. In labour it provides the strong contractions that push the fetus along the birth canal |
|
|
Describe the lower uterine segment. |
This portion lies between the vesico-uterine fold of the peritoneum superiorly and the cervix inferiorly. During pregnancy the upper part of the cervix is incorporated into the lower uterine segment, which stretches to accommodate the fetal presenting part. In late pregnancy as the upper segment muscle contractions increase in frequency and strength, the lower uterine segment develops more rapidly and is stretched radially to permit the fetal presenting part to descend. In labour the entire cervix becomes incorporated into the stretched lower uterine segment. |
|
|
Describe the changes of the cervix uteri. |
In late pregnancy the cervix becomes softer because of chemical changes in the collagen fibres and shorter as it is incorporated into the lower uterine segment. It also undergoes a variable degree of dilatation. Collectively these changes are termed cervical ripening. |
|
|
escribe the path of the fetus into the vagina until it reaches the pelvic floor. |
When myometrial contraction and retraction have led to full dilatation of the cervix the fetal head descends into the vagina which expands to encompass it. As the fetal head descends it encounters the pelvic floor and the leading point is directed forwards by the gutter formed by the levatores ani. |
|
|
How has the vagina adapted to accomodate the fetus? |
The vaginal muscle has hypertrophied and the epithelium become folded during pregnancy, so it is not damaged, it also expands. |
|
|
Once the fetus reaches the pelvic floor what structure must it now pass through? And how does it do this? |
The urogenital diaphragm. The levator muscles stretch and are displaced downwards and backwards, so that the anus receives the full force of the descending head and, dilating, gapes widely to expose the anterior rectal wall. Pressure is also exerted on the lower part of the vagina and the central portion of the perineum. These tissues may tear. |
|
|
How does the fetus exit the uterus? |
The descent is straight to the level of the ischial spines; it then moves in an anterior curve around the lower border of the pubic symphysis. If the pubic arch is wide, the head will stem close behind the symphysis and the perineum will not be so stretched. If the angle is narrow the head is forced back, the direction of the curve is more obtuse and perineal damage is likely. |
|
|
What are the 5 bones that make up the fetal skull? |
2 parietal bones 2 frontal bones Occipital bone |
|
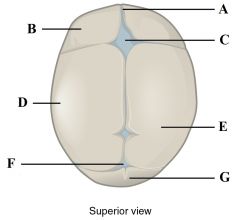
Define labels A - G on the fetal skull. |
A - Frontal suture B - Frontal bone C - Anterior fontanelle D - Ossification centre E - Parietal bone F - Posterior fontanelle G - Occipital bone |
|
|
How does blood change during pregnancy? |
Plasma volume increases to fill the additional intravascular space created by the placenta and the blood vessels The red cell mass increases to meet the increased demand for oxygen Because the increase in the RCM is proportionately less than the increase in plasma volume, the conc. of erythrocytes in the blood falls with a reduction in haemoglobin conc. Even though the haemoglobin conc. falls later in pregnancy a larger total haemoglobin is present than when not pregnant The number of WBCs increases. |
|
|
Describe how the cardiovascular system changes to deal with pregnancy. |
To deal with the increased blood volume and the additional demand for oxygen in pregnancy, CO increases by 30-50%. Most of this is due to increased stroke volume but the heart rate also increases by 15%. The increased CO is balanced by a decrease in peripheral vascular resistance. So blood pressure falls in early pregnancy, rising back to pre-pregnancy levels by the third trimester The veins in the leg become distended - due to the obstruction of venous return caused by the higher pressure of the venous blood returning from the uterus and the mechanical pressure of the venous blood returning from the uterus and the mechanical pressure of the uterus on the IVC - variscosities. |
|
|
What are the changes that occur to the respiratory system during pregnancy? |
Tidal volume and ventilation rate increase. Increased mix of gases and an increased oxygen consumption. This is because of the increased secretion of progesterone. May lead to overbreathing and a lower arterial PO2 |
|
|
What are the changes that occur to the GI system during pregnancy? |
The lower oesophageal sphincter is relaxed - may permit regurgitation of gastric contents and cause heartburn. Gastric secretion is reduced and food remains longer in the stomach. The intestinal musculature is relaxed with lower motility, which permits a greater absorption of nutrients but may lead to constipation. |
|
|
What are the changes that occur to the renal system during pregnancy? |
The smooth muscle of the renal pelvis and ureters relaxes, causing dilatation - this increases the capacity of the renal pelvis and ureters and increases the chance of urinary stasis. Also the bladder musculature relaxes. Renal blood flow increases to the 16th week of pregnancy then levels off. The GFR increases in early pregnancy and remains at the new level until the last 4 weeks of pregnancy when it falls. As tubular reabsorption is unaltered the clearance of many solutes is increased. The increased GFR with the natriuretic effect of progesterone would cause an increased loss of sodium were it not for increased production of renal renin and in consequence angiotensin. |
|
|
What are the changes that occur in the immune system during pregnancy? |
HcG can reduce the immune response of pregnant women. Serum levels of IgG, IgA and IgM decrease from the 10th week of pregnancy, lowest at 30th week then remaining at this level to term - increase in risk of infection |
|
|
How does oestrogen affect blood pressure? |
Oestrogen causes more nitric oxide release from endothelium which stimulates cAMP leading to decreased intracellular calcium conc. and as a result that inhibits contraction of blood vessels - they are wider so resistance decreases - BP lowers. |
|
|
What is the role of nitric oxide in the cardiovascular system? |
Powerful vasodilator - causes SM relaxation, vasodilation and increased blood flow. It works via cGMP and PKG to decrease calcium conc. and cause smooth muscle relaxation. It's precursor is nitroglycerin. |
|
|
Explain the term gravity. |
The number of times a women has been pregnant. |
|
|
Explain the term parity. |
The number of times a woman has given birth. |
|
|
Explain the term primigravida. |
A first time mum. |
|
|
What is dilutional anaemia? |
The difference between the high levels of plasma and low levels of red blood cells is bigger than normal. The hormone EPO is released which causes the differentiation of stem cells into red blood cells but there is a lag between the rise in hormone and the differentiation. So less iron. |
|
|
What are the 5 things that are tested on a newborn baby to produce the APGAR score? |
Activity (muscle tone) Pulse Grimace (reflex irritability) Appearance (skin colour) Respiration |
|
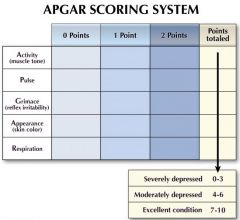
Fill in the APGAR scoring table. |
Activity - Absent - Arms and legs flexed - acative movement Pulse - Absent - under 100bpm - over 100bpm Grimace - Flaccid - some flexion of extremities - active motion (sneeze, cough, pull away) Appearance - blue, pale - body pink, extremities blue - completely pink Respiration - Absent - slow, irregular - vigorous cry |
|
|
What is the role of the parathyroid hormone in pregnancy? |
Is responsible for calcium metabolism control. The foetus requires a lot of vitamin D and calcium. The peak in PTH levels occurs during the 15th-35th week period as this is the time when foetal skeletal growth is at its greatest. |
|
|
How does the mother adapt to provide glucose for the foetus? |
The mother's glucose that she produces crosses the placenta to feed the foetus. The amount of insulin she produces also decreases as insulin is unable to cross the placenta. As well as this, oestrogen, progesterone and cortisol decrease the mother's ability to use the insulin she has produced. This causes a higher amount of glucose to circulate in her blood stream meaning there is more available to flow to the foetus. Less glucose is then reaching the mother's cells - tiredness due to hypoglycaemia. |
|
|
What is hydronephrosis? |
Pregnancy can cause this condition. It's the welling of the kidneys when urine flow is obstructed in any part of the urinary tract. Pregnancy usually causes ureteral obstruction from the pressure of the enlarged uterus on the ureters. |
|
|
What are the functions of progesterone? |
Maintenance of the endometrial lining. Suppresses ovulation Prevents contraction of the myometrium Breast development Prevents immunorejection of the embryo |
|
|
Explain the HPA axis in pregnancy. |
The hypothalamus produces corticotropin releasing hormone. This causes the anterior pituitary to release adrenocorticotropic hormone (ACTH). This causes the adrenal cortex to release cortisol. Usually cortisol negatively feeds back to the level of the anterior pituitary gland. But in pregnancy it positively feeds back so levels of ACTH and cortisol are high. |
|
|
Give a summary of the roles of hCG, hPL and oestrogen in pregnancy. |
hCG - peaks at 8-10 weeks gestation, maintains the corpus luteum, contributes to morning sick hPL - peaks towards end of gestation, involved with the proliferation of mammary tissue and milk secretion & has some metabolic effects Oestrogen - major placental steroid, increases myometrial excitability & initiates labour |
|
|
Give a summary of the roles of progesterone, relaxin and oxytocin in pregnancy. |
Progesterone - maintains the endometrial lining and prevents premature expulsion of the fetus. Relaxin - softens the cervix and pelvic ligaments in preparation for labour. Oxytocin - Responsible for the let down reflex which results in milk ejection due to the suckling stimulus |
|
|
Give a summary of the roles of prolactin, cortisol and thyroid hormones. |
Prolactin - responsible for lactogenesis in the breast alveoli. Inhibited by the major placental steroids and dopamine Cortisol - a long term stress hormone which has some metabolic effects during pregnancy - responsible for fat deposition & stretch marks Thyroid hormones - high levels of T3 and T4 peak gestation and may have some involvement with morning sickness. |
|
|
What is the first stage of pain in labour? |
Onset to cervical dilation Includes: Repetitive uterine contractions - innervation of the uterus is by C fibres - small and unmyelinated - accompany the sympathetic nerve endings and travel in the paracervical ganglion, hypogastric plexus and lumbar sympathetic chain Distension of lower uterine segment also occurs. Nerves involved T10-L1 |
|
|
What is the second stage of pain in labour? |
Full cervical dilation to delivery of the baby. Includes: Repetitive uterine contractions Distension of vagina, pelvic and perineal structures Still have C fibres going to lumbar plexus (T10-L1) But also has fast myelinated A delta fibres going to the lumbo sacral plexus, pudenal nerves - sharp pain - localised |
|
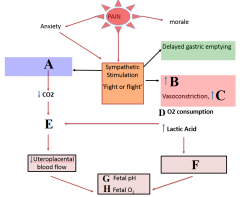
Define labels A - H |
A - hyperventilation B - cardiac output C - blood pressure D - decrease E - uterine artery vasoconstriction F - Maternal metabolic acidosis G - decrease H - decrease |
|
|
What are the factors influencing pain in labour? |
Parity Anatomy Physiology Psychology Position of fetus Size of fetus Analgesia |
|
|
What factors create the perfect analgesia for labour? |
Effective pain relief No risk of harm to mother and baby No impact on progress of labour No systemic effects Does not cross the placenta Immediately titratable |
|
|
What are the analgesic options for women in labour? |
Non-pharmacological Pharmacological Neuraxial (spinal cord) Nerve blocks (peripheral nerves) |
|
|
Describe pain transmission in the dorsal horn. |
The afferent neurons enter dorsal horn They synapse in the substantia gelatanosa Interneurones mediate sensitivity of secondary afferents Secondary afferents cross and ascend in spinothalamic tract |
|
|
Explain the spinal cord gate mechanism. |
Alpha beta fibres - mechanoreceptors respond to pressure and touch C fibres - nociceptive, respond to pain and temp They converge on inhibitory interneurone gates - pain transmission at dorsal horn. When unopposed C fibres stimulate secondary afferents at dorsal horn, inhibitory neurones are surpressed. When alpha beta fibres are stimulated, inhibitory neurones are activated so there is reduced transmission of pain signals to afferents - c fibres cant get through - pain gate is closed - massage helps |
|
|
Why should you avoid NSAIDS in labour? |
Because the fetus' ductus arteriosus is dependent on prostaglandins |
|
|
What are the 3 pharmacological options for pain relief in labour? |
Paracetamol Entonox (laughing gas) Opiates
|
|
|
Describe what entonox is and a bit about how it works. |
Entonox is 50:50 oxygen : nitric oxide It has a rapid on/off onset Has no effect on APGAR scores Can make you feel sick It also interferes with B12 metabolism in the body (inactivates B12) - not bad for healthy pregnant women Makes you feel euphoric, sleepy |
|
|
Describe what opiates are and how they work. |
Opiod alkaloids are active at opiod receptors Mu, Kappa and Delta. They are widespread in the CNS. - tractus solitaries, PAG, cerebral cortex, thalamus, and substantia gelatinosa. The receptors are G-protein coupled. Hyperpolarised neurons decreases excitability. Euphoria, sedation, nausea, respiratory depression Given via IV or IM. Has neonatal effects - effects APGAR score Naloxone - competitive antagonist at u-opiod receptor |
|
|
Give 4 opiates and which is the most common opiate given IM? |
Morphine, diamorphine, remifentanil and most common: pethidine
Pethidine is metabolised to norpethidine which has half life of 20 hours for mum and 60 for baby.
|
|
|
Give the steps in a neuraxial epidural and its effects. |
Epidural - insert needle into: skin subcut tissue supraspinous ligament interspinous ligament ligamentum flavum epidural space causes vasodilation outside dura - slow onset but has a catheter so prolonged effect minimal foetal transfer of drugs no sedation |
|
|
Give the steps in a neuraxial spinal and it's effects. |
Spinal - insert needle into: skin subcut tissue supraspinous ligament infraspinous ligament ligamentum flavum epidural space dura aspirate CSF causes vasodilation The anaestetic solutionis injected into the spinal fluid rapid onset - dense block quicker to work than epidural no catheter - may not last long enough for labour minimal foetal transfer of drugs below L1-2 |
|
|
Give the neuraxial contraindications. |
Patient refusal Abnormal spinal anatomy (spina bifida, lumbar fusion) Impaired coagulation (risk of spinal haematoma) Infection (risk of spinal meningitis/abscess) Fixed CO state - severe aortic stenosis, massive haemorrhage - don't want to vasodilate as they will lose their BP.
|
|
|
Give the complications of neuraxials. |
Hypotension - sympathetic block, vasodilation Failure - incorrect placement Dural puncture headache - CSF leak - low pressure headache nerve damage - direct/indirect Meningitis - CNS infection Local anaesthetic toxicity - toxic systemic levels of anaesthetic High block - paralysis of respiratory muscles |
|
|
How do neuraxials affect labour? |
Need blood pressure and pulse constantly monitored May cause motor block - heavy legs Reduced urge to push (reduced sensation) - prolonged second stage - more chance of instrumental delivery no increase in caesarean rate no increase in long term backache |
|
|
Which nerves do nerve blocks target? |
Nerve blocks target the peripheral nerves not spinal nerves. Particularly the pudenal nerves S2,3 and 4 ( just behind the sacrospinous ligament) Used for perineal anaesthesia in second stage labour, instrumental deliveries, suturing and tissue infiltration.
|
|
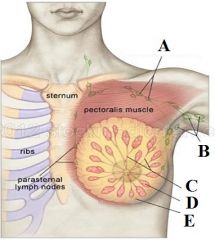
Define labels A - E |
A - Apical lymph nodes B - Axillary lymph nodes C - Breast tissue ducts D - Gland (lobe) E - Fat |
|
|
What is the breast supported by? |
A framework of fibrous connective tissue called Cooper's ligaments. |
|
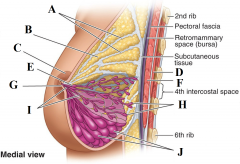
Define labels A - J |
A - Suspensory ligaments B - Fat lobule C - Areola D - Pectoralis minor E - Lactiferous sinus F - Pectoralis major G - Nipple H - Lobules of mammary gland (resting) I - Lactiferous ducts J - Lobules of mammary glands (active) |
|
|
What happens to the breast anatomy during puberty? |
Before puberty the breast consists of lactiferous ducts. During puberty these ducts sprout and become highly branched. This is under the influence of the ant. pituitary, ovarian and adrenal hormones (growth hormone, progesterone and oestrogen) |
|
|
What happens to the breast anatomy during menstruation and then pregnancy? |
Menstruation - progesterone stimulates the formation of spherical masses of granular cells at the end of each duct Pregnancy - immature alveoli develop into milk secreting alveoli during pregnancy |
|
|
Describe the lobe and lobule system. |
Glandular tissue is composed of lobes that comprise of lobules. Each breast is divided into 15-20 lobes by fibrous septa that radiate from the centre. 1 lactiferous duct drains a lobe. Each lobule contains a certain number of alveoli and ducts - not uniform in shape, size and number.
|
|
|
Describe the ejection of milk at the alveoli level. |
The alveoli contain acini cells (columnar epithelium), which produce milk and are surrounded by myoepithelial cells. There is a dense network of capillaries surrounding the alveoli. Contraction of myoepithelial cells squeezes th alveoli and ejects the milk into the larger duct. |
|
|
Describe the path of milk from the lobule duct to the opening of the nipple. |
The lobule ducts connect with larger ones called lactiferous ducts. One large duct leaves each lobe and widens to form a lactiferous sinus (or ampulla). A lactiferous tubule from each sinus opens on the surface of the nipple. |
|
|
Describe the nipple. |
The nipple is a muscular projection covered by pigmented skin. Longitudinal and horizontal smooth muscle fibres. Sphincter like function. 23-27 ducts at base of nipple.
|
|
|
What are the main arteries and veins that supply the breast? |
The mammary artery and its branches - Lateral thoracic artery Intercostal arteries
Veins - Intercostal veins - internal mammary vein - axillary vein - superior vena cava
|
|
|
What is the nerve supply of the breast? |
The 4th, 5th and 6th intercostal nerves |
|
|
What are Montgomery's tubules? |
Sebaceous glands around the nipple become hypertrophied. The keep the nipple and aerola moist and healthy. |
|
|
What are the 4 phases in the physiology of lactation? |
Mammogenesis - preparation of breasts Lactogenesis - synthesis and secretion from the breast alveoli Galactokinesis - Ejection of milk Galactopoiesis - Maintenance of lactation |
|
|
What occurs during stage 1 of lactogenesis? |
There is an increase in mRNA for milk proteins and enzymes. Fat droplets increase in size. Production of milk specific proteins - lactose casein and alpha-lactalbumin these are reabsorbed into the blood. Full milk secretion is prevented by high levels of progesterone and oestrogen. |
|
|
What is the cue for stage 2 of lactogenesis and what does it involve? |
At birth there is a sudden drop in oestrogen, progesterone and hPL. This and the high levels of prolactin are a cue for lactogenesis. Need placenta out for it to happen. Theres an increase in lactose, IgA and lactoferrin (blocks bacteria taking iron). There is an increase in milk levels and oligosaccharide levels (protective against pathogens) This process is hormonally driven and milk removal is necessary by day 3 to continue lactation |
|
|
What change occurs after lactogenesis 2? |
Control switches from endocrine to autocrine (local) control. |
|
|
What are the most important factors for galactopoeisis? |
Prolactin is the most important galactopoietic hormone. Therefore continuous suckling is essential for galactopoeisis - removes milk so more can be made and stimulates prolactin release. The whey protein FIL (feedback inhibitor of lactation) regulates milk secretion. Milk production speeds up when the breast is emptier. Fat content dependent on how empty the breast is - emptier breast = higher fat content |
|
|
Describe the mechanisms behind Galactokinesis. |
Depends not only on the suction exerted by the baby during suckling but also on the contractile mechanism which expresses the milk from the alveoli into the ducts. The contractile mechanism is under the control of the hypothalamus, posterior and anterior pituitary and its neural reflex arc. |
|
|
Outline the neural pathways the produces the "milk let down reflex". |
Suckling stimulates mechanoreceptors in nipple, The nerve impulse travels to the hypothalamus, This travels down the nervous pathway, This travels to the posterior pituitary, It releases oxytocin, This stimulates the contraction of myoepithelium cells surrounding alveoli, Milk ejection |
|
|
How does oxytocin cause myoepthelial cells to contract? |
Interaction of oxytocin with its receptors raises the level of intracellular calcium. This allows the myoepithelial cells to contract. |
|
|
What is the composition of human milk in the first week after birth? |
It is called colostrum. It is sticky, yellowish, relatively low in fats and lactose. It is rich in proteins, minerals and fat soluble vitamins - A,D,E and K Contains immunoglobins Has a laxative effect |
|
|
What is the composition of mature milk? |
At 3 weeks milk is no longer colostrum. The calorific value increases. High in fats, sugars and amino acids. |
|
|
What are the benefits of milk sugars and milk proteins? |
The milk proteins: casein, alpha-lactalbumin and lactoglobulin help produce intestinal flora. Lactose promotes growth of intestinal flora Cows milk contains way more casein (curds) than human milk which is harder for babys to digest. |
|
|
What is the main immunoglobulin in breast milk? |
IgA - protects against pathogens and prevents colonisation |
|
|
How do immunoglobulins protect infants? |
They directly bind to specific microbial antigens, they block, bind and stick to them, they enhance phagocytosis, they modulate local immune function, they contribute to the infant's immune system development. |
|
|
How does lactoferrin in breast milk benefit the infant immunologically? |
Chelates iron, it blocks the absorption/penetration of viruses and adhesion of bacteria, Contributes to intestinal growth and repair, decreases the production of inflammatory cytokines |
|
|
How do lysozymes, alpha-lactalbumin and casein benefit the infant immunologically? |
Lysozyme - causes bacterial cell wall lysis, binds endotoxin, increases IgA production and contributes to macrophage activation. lactalbumin - promotes growth of good bacteria like bifidobacterium. Casein - inhibits adhesion of various bacteria in different epithelial sites and promotes growth of bifidobacterium |
|
|
What is the pneumonic for the mechanism of labour? |
Do Frogs In Cardiff Ever Ride In A Pink Limo D - Descent F - Flexion (baby tucks chin into chest) I - Internal rotation of head C - Crowning E - Extension R - Restitution (rotation of head back to original position) I - Internal rotation of shoulders A - Anterior shoulder P - Posterior shoulder L - Lateral flexion |
|
|
What is the first stage of labour? |
Bewtween the first onset of regular contractions and full dilatation of the cervix. Before full dilatation happens the shape of the cervix changes. The bulb of the cervix flattens (effacement). The cervix has to be dilated to 3cm. |
|
|
What is the second stage of labour? |
Occurs between complete dilatation (10cm) until the baby is born. |
|
|
What is the 3rd stage of labour? |
Delivery of placenta. |
|
|
What drug can be given to speed up the 3rd stage? |
Syntometrine (ergometrine maleat and oxytocin). this reduces the 3rd stage from 30 to 5 minutes, reducing the risk of post-partum haemorrhage. |
|
|
What are the 3 signs of placental separation? |
Cord lengthening Gush of blood Uterus clamps down (returns to a smaller size) |
|
|
Give 4 common complications in labour. |
Labour distosia - inadequate power - contractions aren't strong enough - baby can't get through Brachial plexus paralysis - when you pull the babys head, can give them waiter's tip because C5, C6 and C7 are affected & from pulling arm Cord prolapse - umbilical cord prolapses into the vagina before the fetus, when fetus descends it could choke Shoulder dystocia - the shoulder hits the pubic symphysis - gets stuck |

