![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
39 Cards in this Set
- Front
- Back
|
Elements |
Only made out of one type of atom. All the elements we have found are in the Period Table each their own chemical symbol |
|
|
Compound |
When two or more atoms chemically bond together. E.g Sodium Oxide - Na2O |
|
|
Atom |
The smallest piece of a atom that can exist |
|
|
Mass number |
The top number The total amount of protons and neutrons because they are the only subatomic particles that have a mass. |
|
|
Atomic Number |
The bottom number The total amount of protons |
|
|
Nucleus |
The nucleus of the atom contains protons and neutrons Electrons orbit the nucleus in shells |
|
|
Protons |
Charge of +1 Found in Nucleus Mass of 1 |
|
|
Electrons |
Charge of -1 Orbit the nucleus in shells Mass of 0 Can move out of element to bond with others |
|
|
Shells around the nucleus |
Contain electrons 1st shell - max 2 electrons 2nd shell - max 8 electrons 3rd shell - max 8 electrons |
|
|
Chemical Equations |
Show reactants on the left and products on the right Word equations and symbol equations No atoms are created or destroyed so the number of atoms will remain the same throughout the equation. (This is called balancing an equation) State symbols (aq) - aqueous (in water) |
|
|
Law of Conservation of Mass |
States that no atoms are lost or made during a chemical reaction, so the mass of the products equals the mass of the he reactants. |
|
|
Mixture |
Made up of two or more substances (elements or compounds) that are not chemically bonded together. The chemical properties of the products are kept the same. They can be separated |
|
|
Filtration |
Separates insoluble solids and liquids in a solvent I.e sand and water. Performed by: - Pouring a mixture through filter paper - The insoluble solid is trapped by the filter paper - The liquid runs through the filter paper and is collected below |
|
|
Crystallisation |
Separates soluble solids in a solvent. Performed by: - Heating a mixture so the solvent evaporates - Eventually, the crystals of the solute (dissolved solids) will form - We can collect the solvent by condensing it as it evaporates |
|
|
Chromatography |
Separates solutions with a number of different solutes (solids) in a solvent (liquids). - Place a drop of the solution near the bottom of a piece of chromatography paper. - Dip the bottom in a suitable solvent. The solvent moves up the paper and carries the solutes (solids) in the solution with it |
|
|
Solute |
A solid dissolved in a liquid |
|
|
Solvent |
The liquid that solids dissolve into |
|
|
Solution |
A mixture of a solid and a liquid |
|
|
Why does Chromatography work? |
Because some substances are more soluble then others. This means they will move up the paper more |
|
|
Miscible liquids |
Dissolve in each other, mixing completely. Do not form separate layers Fractional distillation is used to separate miscible liquids |
|
|
Fractional Distillation |
Used to separate liquids with different boiling points. The mixture is slowly heated in a fractionating column with the lowest boiling point is collected first. Used to separate crude oil and ethanol from a fermented mixture in the alcoholic drinks industry. |
|
|
Atomicity |
The amount of an atom there is Represented in the bottom right corner of the atom. |
|
|
Simple Distillation |
Separates 2 liquids with different b.p - mixture is heated until liquid with lower b.p boils - the vapour passes through a condenser and turns back into a liquid on the other side. Can also separate a solute from a solvent , when the solvent has a low b.p than the solute. |
|
|
How did J.J Thompson contribute to the construction of the Atom? |
Discovered electron Proposed ‘plum pudding model’ - a ball of positive charge with a negatively charged electrons embedded in it. |
|
|
How did Geiger and Marsden contribute to the construction of the atom? |
They did experiments firing alpha particles at a piece of gold foil. They concluded their must be a tiny spot of positive charge in the centre of an atom. |
|
|
Plum Pudding Model |
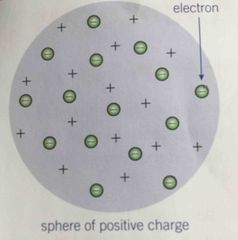
Back (Definition) |
|
|
Nuclear Model |
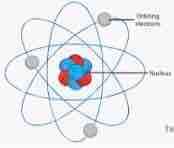
Back (Definition) |
|
|
How did Rutherford contribute to the construction of the atom? |
He proposed the nuclear model based on Geiger and Marsden’s experiments. In this model electrons orbit around a nucleus which contains protons. |
|
|
How did Bohr contribute to the construction of the atom? |
Bohr discovered that electrons orbit the nucleus in energy shells in a set distance. He revised the nuclear model to match these experimental observations |
|
|
How did Chadwick contribute to the construction of the atom? |
He showed the existence of uncharged particles called neutrons in the nucleus. |
|
|
Neutrons |
Charge of 0 Mass of 1 |
|
|
Isotopes |
Every atom of the same element contains the same number of protons, but isotopes are atoms of the same element that have different number of neutrons. |
|
|
Protium |
Isotope of Hydrogen 1 proton and 0 neutrons. Used in Hydrogen Fuel Cells and Making Plastic. 99.98% of hydrogen atoms are protium |
|
|
Deuterium |
Isotope of hydrogen Used in nuclear fusion Around 0.02% of hydrogen atoms are deuterium |
|
|
Trituim |
Isotope of Tritium Contains 1 proton and 2 neutrons Very rare Used in thermonuclear fusion weapons |
|
|
Relative Atomic Mass |
The average mass of all the isotopes of an element, taking into account how often each isotope is found (isotope abundance |
|
|
Relative Atomic Mass |
The average mass of all the isotopes of an element, taking into account how often each isotope is found (isotope abundance |
|
|
How do you calculate Relative Atomic Mass? |
Sum of isotope abundance x Isotope mass number / Isotope abundance I.e 2 isotopes 75% isotope 1, mass number = 35 25% isotope 2, mass number = 37 (0.75 x 35) + (0.25 x 37) / 1 = 35.5 |
|
|
Ion |
Charged atom (or group of atoms) Gains electrons, negative ion Loses electrons, positive ion |

