![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
109 Cards in this Set
- Front
- Back
|
..
|
|
|
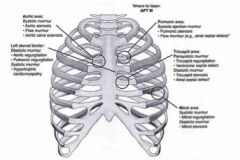
Name the organs in the vessel rich group. What percent of cardiac output goes to each of these organs?
|
• The brain, kidney, liver, lungs, heart, digestive tract and endocrine tissues are organs of the vessel rich group (VRG). These are the well perfused organs.
• % of the cardiac output › 4-5% heart › 15% brain › 20% kidneys › 25 % liver › 100% lungs |
|
|
What percentage of cardiac output is delivered to the highly-perfused organs (heart, lungs, brain, kidneys, and liver)?
|
• Approximately 75% of resting cardiac output is delivered to the vessel rich organs, although they constitute only 10% of total body mass.
|
|
|
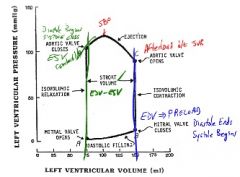
In words, describe where isovolumetric relaxation occurs on the left ventricular pressure-volume loop.
|
• Isovolumetric relaxation occurs from closure of the aortic valve to opening of the mitral valve on the left ventricular pressure-volume loop.
|
|
|
In words, describe where isovolumetric contraction occurs on the left ventricular pressure-volume loop.
|
• Isovolumetric contraction occurs from closure of the mitral valve to opening of the aortic valve on the left ventricular pressure-volume loop.
|
|
|
..
|
|
|
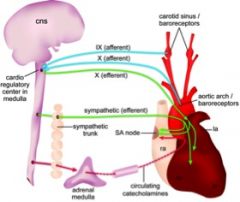
What nerves carry the afferent and efferent signals of the Bainbridge reflex? What does the Bainbridge reflex help prevent?
|
• When the great veins and right atrium are stretched by increased vascular volume, stretch receptors send afferent signals to the medulla via the vagus nerve. The medulla then transmits efferent signals via the sympathetic nerves to increase heart rate (by as much as 75%) and myocardial contractility. The Bainbridge reflex helps prevent damming up of blood in veins, the atria, and the pulmonary circulation.
|
|
|
..
|
|
|
Describe myocardial preconditioning.
|
• Myocardial preconditioning is a short-term rapid adaptation to brief ischemia such that during a subsequent, more severe ischemic insult, myocardial necrosis is delayed. The infarct-delaying properties of ischemic preconditioning have been observed in all species studied.
• Five minutes of ischemia is sufficient to initiate preconditioning, and the protective period lasts for 1 to 2 hours. |
|
|
Describe the cellular mechanisms mediating myocardial preconditioning.
|
• Pharmacological activation of adenosine receptors (particularly a1 and a2 subtypes) initiates preconditioning via intracellular signal transduction mechanisms involving protein kinase C and adenosine triphosphate (ATP)-dependent potassium channels (KATP). Other factors involved include including the sodium: hydrogen exchanger, inhibitory G proteins, and tyrosine kinase.
|
|
|
What anesthetic agents can trigger or modulate the myocardial preconditioning response?
|
• The volatile anesthetics mimic ischemic preconditioning and trigger a similar cascade of intracellular events resulting in myocardial protection that lasts beyond the elimination of the anesthetic. Adenosine or opioid agonists delivered into the coronary circulation may also mimic preconditioning.
• Ketamine antagonizes the protective effect of preconditioning and thus should be used with caution in the patient at risk for myocardial infarction in the perioperative period. |
|
|
What monitoring is indicated for managing the patient with a history of congestive heart Failure secondary to diastolic dysfunction?
|
• The use of invasive monitoring such as central venous pressure (CVP) or pulmonary artery catheter (PAC) may be indicated in managing the patient with a history of congestive heart failure secondary to diastolic dysfunction.
|
|
|
List five (5) compensatory responses in the patient with cardiac failure.
|
• Four major compensatory mechanisms participating in the response to cardiac failure are
› (1) increased left ventricular preload › (2) increased sympathetic tone › (3) activation of the renin-angiotensin-aldosterone system › (4) release of AVP (arginine vasopressin, antidiuretic hormone) › (5) ventricular hypertrophy • These mechanisms initially compensate for cardiac failure, but with increasing severity of the disease, they may actually contribute to the cardiac impairment. • The RAA and sympathetic nervous system contribute to the progressive structural changes in the peripheral vasculature and in the remodeling of the left ventricle. |
|
|
What is the principal hemodynamic alteration with cardiac tamponade?
|
• The principal hemodynamic feature of cardiac tamponade is a decrease in cardiac output due to a reduced stroke volume, secondary to an increased central venous pressure and thus reduced venous return to the heart.
• The diagnosis of postoperative cardiac tamponade should be considered whenever hemodynamic deterioration is encountered, particularly when reductions in CO or BP or both are not readily resolved by conventional management. |
|
|
What is Becks Triad?
|
• Becks triad is the constellation of:
› (1) Hypotension › (2) Jugular Venous Distension › (3) Distant, muffled heart sounds |
|
|
What muscle acts as a barrier to regurgitation in the conscious subject?
|
• In the awake subject, the cricopharyngeus muscle is the primary muscular barrier to regurgitation.
|
|
|
Laryngospasm is caused by stimulation of which nerve?
|
• Stimulation of the superior laryngeal nerves may cause laryngospasm.
• Note: external branch of SLN innervates the crycothyroid muscle. |
|
|
..
|
|
|
West's zones of the lung describe alveolar perfusion based on the relationship of three pressures: alveolar pressure (PA), arterial pressure (Ppa) and venous pressure (Ppv). Which region shows the greatest increase in blood flow over the distance of the zone?
|
• West zone 2 shows the greatest increase in blood flow over the distance of the zone; blood flow is zero at the nondependent start of zone 2 and increases with dependency over the distance of zone 2.
• Reminder: zone 2 is the "waterfall zone" of intermittent blood flow, where Ppa > PA > Ppv |
|
|
West's zones of the lung describe alveolar perfusion based on the relationship of three pressures: alveolar pressure (PA), arterial pressure (Ppa) and venous pressure (Ppv). Which region maximal blood flow of any zone?
|
• West zone 3 possesses the maximal pulmonary blood flow of any zone (region).
• Reminder: zone 3 is the "distension zone" with continuous blood flow, where Ppa > Ppv > PA. |
|

|
...
|
|
|
How does marked right-to-left intrapulmonary shunting manifest on radiographs?
|
• Marked right-to-left intrapulmonary shunting (shunt flow >15%) is associated with radiographically discernable findings such as pulmonary atelectasis, parenchymal infiltrates, or a large pneumothorax.
|
|
|
What happens to pulmonary blood flow in zone 4 of the lung?
|
• Lung regions where Ppa>PISP>Ppv>PA are termed zone 4 regions.
• Blood flow in zone 4 is reduced by gravitational compression of the lung parenchyma or by interstitial edema formation. |
|
|
What are pulmonary J-receptors?
|
• Juxtapulmonary-capillary receptors (J receptors) are located in the walls of the pulmonary capillaries or in the interstitium, hence the name. J receptors appear to be stimulated by pulmonary vascular congestion or an increase in pulmonary interstitial fluid volume, leading to tachypnea. The J receptors may also be responsible for the dyspnea encountered during pulmonary vascular congestion and edema secondary to left ventricular failure.
|
|
|
Which nerve fiber type innervates pulmonary J receptors?
|
• C-fibers lie in close relationship to the pulmonary microcirculation and appear to innervate the pulmonary J receptors.
• The afferent pathway from the J receptors is the slow-conducting nonmyelinated (C) fibers in the vagal nerves. |
|
|
Lung auscultation reveals basilar crackles and chest radiographs exhibit "whited-out" areas; what is your diagnosis?
|
• The detection of basilar crackles on auscultation is the traditional hallmark of early pulmonary edema.
• A "butterfly" appearance or "whitedout" areas on chest radiographs support the diagnosis of pulmonary edema. |
|
|
What is the primary mechanism of hypoxemia in the patient with chronic obstructive pulmonary disease COPD)?
|
• The primary mechanism of hypoxemia in obstructive pulmonary disease is regional mismatch of ventilation and perfusion (VIQ mismatch).
• COPD → V/Q mismatch → hypoxemia |
|
|
Which neurotransmitter is the most common excitatory neurotransmitter in the central nervous system (CNS)?
|
• Glutamate is the most common excitatory neurotransmitter in the central nervous system (CNS).
• Glutamate is an excitatory amino acid neurotransmitter. |
|
|
List three (3) common ionotropic glutamate receptors in the central nervous system (CNS). Which electrolytes (ions) pass through these receptors upon activation?
|
• The three ligand-gated ionotropic glutamate receptors of the CNS are:
› (1) N-methyl-D-aspartate = NMDA › (2) AMPA › (3) kainate • When the ligand glutamate binds to these ionotropic receptors, a transmembrane, cation-selective channel opens, permitting influx of Na+ and Ca2+ and efflux of K+. • Sodium is the main ion permeating the channel, leading to membrane depolarization. |
|
|
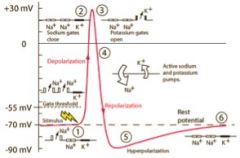
What membrane ion channels play a role in cell repolarization?
|
• The necessary actor in causing both depolarization and repolarization of the nerve membrane during the action potential is the voltage-gated sodium channel. A voltage-gated potassium channel also plays an important role in increasing the rapidity of repolarization of the membrane.
• Repolarization begins with the closing of the voltage-gated sodium channels, followed by opening of the voltage-gated potassium channels. During early repolarization, the sodium channels are in the closed, inactive conformation causing the cell to be absolutely refractory to stimulus. During the latter stages of repolarization, the voltage-gated sodium channels have returned to the closed, resting conformation, and the cell is relatively refractory to stimulus. |
|
|
..
|
|
|
What is the conus medullaris? What is the filum terminale?
|
• The conus medullaris is the blunt, tapering tip of the spinal cord. The pia alone continues from the conus medullaris and after piercing the dural sac, continues with a covering of dura to the coccyx, forming the filum terminalis.
• The filum terminalis is comprised of the pia and dura mater. |
|
|
Describe the anatomy of the hypogastric plexus.
|
• The pelvic viscera in men and women -- the urogenital organs, the colon, and the rectum -- are supplied by afferent fibers from the lumbar sympathetic chain.
• The superior hypogastric plexus is a retroperitoneal structure that is formed by confluence of the bilateral lumbar sympathetic chains; it is situated between the bodies of the L5 and S1 vertebrae. The pelvic pain caused by either inflammatory diseases or cancer can be relieved by interruption of bilateral sympathetic pathways, which can be achieved with a superior hypogastric plexus block. |
|
|
Where do preganglionic parasympathetic nerves originate?
|
• Preganglionic parasympathetic nerves arise from
› nuclei of cranial nerves III, VII, IX and X (CN 2, 7, 9, 10) in the brainstem › sacral segments 2-4 (S2-S4) of the spinal cord. • Owing to these origins, the parasympathetic system is also known as the craniosacral division. |
|
|
Intracranial hypertension occurs with a sustained increase in intracranial pressure (ICP) above 15 to 20 mm-Hg. Above what ICP will a 'vicious cycle' of ischemia and edema ensue?
|
• When intracranial pressure (ICP) exceeds 30 mmHg, cerebral blood flow progressively decreases and a vicious cycle is established: ischemia produces cerebral edema, which in turn increases ICP, and further precipitates ischemia. If this cycle remains unchecked, progressive neurologic damage or catastrophic herniation may result.
• ICP > 30mmHg →decreased CBF → ischemia → cerebral edema → increase ICP |
|
|
Identify the anesthetic agents that are absolutely contraindicated in the patient with a family history of malignant hyperthermia.
|
• All volatile (potent) inhalational agents and succinylcholine are absolutely contraindicated in patients with malignant hyperthermia
|
|
|
What is the prophylactic blood level for dantrolene?
|
• The prophylactic blood level for dantrolene is 2.5 mcg/ml.
|
|
|
What is the dosage for dantrolene?
|
• 2.5 mg/kg over 5 minutes until episode is terminated
• Max 10-20 mg/kg |
|
|
How much dantrolene is found in each vial of dantrolene? How must each vial of dantrolene be prepared?
|
• Each vial of dantrolene contains 20 mg with 3 g of mannitol; the vial should be mixed with 50 mL of warmed, bacteriostatic sterile distilled water (not saline solution).
• It is important to store sterile water in clearly labeled containers of a different size from that used for routine intravenous solutions. • Mixture: 20 mg Dantrolene + 50 mL sterile H2O |
|
|
You have successfully treated an acute episode of malignant hyperthermia with dantrolene sodium. Dantrolene should be re-administered at what intervals for how often?
|
• The half-life of dantrolene is approximately 10 hours, therefore dantrolene should be re-administered at least every 10 to 15 hours for at least one and possibly several doses.
• Barash and Stoelting say dantrolene should be supplemented every 6 hours for at least 24 hours following the clinical episode. • Summary: › Half-life: 10 hrs › re-administer every: 10-15 |
|
|
During the preoperative assessment of the patient, you discover a positive family history for malignant hyperthermia. What six steps need to be taken to manage this patient? Are nondepolarizing muscle relaxants safe in this patient?
|
• (1) Anesthesia and premedication should be designed to produce a low normal heart rate. Standard premedicant drugs such as opioids, benzodiazepines, ataractics, barbiturates, antihistamines, and anticholinergics do not cause problems in MH-susceptible patients when administered in appropriate doses,
• (2) the anesthesia machine is prepared by draining, removing, or disabling anesthetic vaporizers, and changing tubing and CO2 absorbent and flowing oxygen at 10 L/min for 10 minutes or longer, • (3) measure end-tidal CO2, • (4) body core temperature should be monitored by nasopharyngeal, rectal, or esophageal routes in all patients for all surgical procedures, • (5) If possible, a regional, local, or major conduction anesthetic should be used. If not possible, intravenous induction of anesthesia followed by nitrous oxide, oxygen, and a nondepolarizing relaxant with opioid supplementation is recommended. Agents that have not been implicated in MH are midazolam, dexmedetomidine, diazepam, droperidol, and propofol, • (5) arterial and central venous monitoring is recommended for MH-susceptible patients, only as dictated by the surgical procedure and • (6) observe patient closely in the postoperative period. |
|
|
What syndrome can mimic malignant hyperthermia?
|
• Neuroleptic malignant syndrome (NMS) has many features in common with malignant hyperthermia but the mode of onset and recovery are quite different.
• MS typically develops over 24 to 72 hours, whereas malignant hyperthermia develops acutely. • NMS presents as a hypermetabolic episode consisting of hyperthermia, autonomic nervous system instability, pronounced muscle rigidity, and rhabdomyolysis. |
|
|
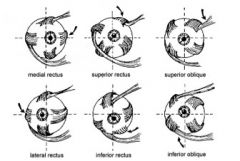
List the six (6) orbital muscles, their function, and their motor innervation.
|
Superior rectus
-Supraduction of orbit -("look up") -CN III (oculomotor) Inferior rectus -Infraduction of orbit -("look down") -CN III (oculomotor) Medial rectus -Adduction of orbit -(“look inward”) -CN III (oculomotor) Lateral rectus -Abduction of orbit -“look outward” -CN VI (abducens) Superior oblique Intorsion depression of orbit -“look in and down” -CN IV (trochlear) Inferior oblique Extorsion elevation of orbit -("look out and up") -CN III (oculomotor) |
|
|
..
|
|
|
Patients treated with what drugs are susceptible to neuroleptic malignant syndrome (NMS)?
|
• Neuroleptic malignant syndrome (NMS) results from treatment with a variety of antipsychotic agents, including phenothiazines, haloperidol and other potent antipsychotics.
• NMS following antipsychotic drug therapy presumably reflects dopamine depletion in the CNS. |
|
|
What are the clinical manifestations of myotonic dystrophy (Steinert's disease)?
|
• Myotonia dystrophy (Steinert's disease, myotonic atrophica) is a multisystem disease that usually manifests as facial weakness (expressionless facies), wasting and weakness of the sternocleidomastoid muscles, ptosis, dysarthria, dysphagia, and inability to relax the handgrip (myotonia).
• Frontal balding, cataracts, and testicular atrophy in males form a frequently recognized triad of characteristics. |
|
|
Identify the genetic pattern of inheritance for myotonic dystrophy and describe the pathophysiology of this group of degenerative diseases.
|
• Myotonic dystrophy (MD) is inherited as an autosomal dominant trait and usually manifests in the second or third decade of life. Myotonic dystrophies are characterized by persistent contracture (myotonia) after voluntary contracture of skeletal muscle or following electrical stimulation.
• Electromyographic findings are diagnostic and are characterized by prolonged discharges or repetitive action potentials. Skeletal muscle resting membrane potentials are also lowered (less polarized) in patients with MD. • The inability of the skeletal muscle to relax after voluntary contraction or stimulation results from abnormal calcium metabolism -- ATP-driven pumps fail to return calcium to the sarcoplasmic reticulum (SR) thus the unsequestered calcium remains available to produced sustained skeletal muscle contraction. |
|
|
What four anesthetic concerns should you have for the patient with myotonic dystrophy?
|
• Preoperative evaluation and management of anesthesia in patients with myotonic dystrophy must consider the likelihood of
› (1) cardiomyopathy › (2) respiratory muscle weakness and sensitivity to respiratory depressants › (3) vulnerability to aspiration of gastric contents › (4) potential for abnormal responses to anesthetic drugs. |
|
|
Should succinylcholine be used in the anesthetic management of the patient with myotonic dystrophy?
|
• Succinylcholine should not be used in the patient with myotonic dystrophy because succinylcholine can produce intense generalized myotonic contracture that makes ventilation or intubation difficult or impossible.
|
|
|
What is the medical management of the patient with myotonic dystrophy?
|
• Treatment of myotonic dystrophy is symptomatic and may include the use of phenytoin, quinine, and procainamide. These agents delay the return of membrane excitation by blocking rapid sodium (Na') influx into muscles. Quinine and procainamide should be used with caution as they may worsen cardiac conduction abnormalities (prolonged P-R interval).
|
|
|
Myasthenia gravis is characterized by what symptoms? What is the cause of these symptoms?
|
• Myasthenia gravis is characterized by weakness and easy fatigability of skeletal muscles.
• The weakness can be asymmetric, confined to one group of muscles, or generalized. • Easy fatigability of skeletal muscle in myasthenia gravis is caused by autoimmune destruction of nicotinic acetylcholine receptors at the neuromuscular junction. |
|
|
Onset of myasthenia gravis (MG) is slow and insidious and any skeletal muscle group may be involved. Onset is most common in which muscles?
|
• The most common onset of myasthenia gravis is ocular.
Ptosis and diplopia result. • If the disease remains localized to the eyes for 2 years, the likelihood of progression to generalized MG is low. |
|
|
What is the best initial test of thyroid function in ambulatory individuals?
|
• In ambulatory individuals, serum TSH is currently the best initial screen for thyroid function.
|
|
|
Calcitonin is released from what organ? Is calcitonin a weak or a strong regulator of calcium?
|
• Calcitonin is a polypeptide hormone secreted from the parafollicular cells (C cells) of the thyroid gland.
• Calcitonin has a quantitatively weak role in calcium homeostasis in the adult. |
|
|
What is the major physiologic action of calcitonin?
|
• Calcitonin tends to decrease plasma concentration of calcium ions, due to a decreased activity of osteoclasts (bone breakdown) and an increased activity of osteoblasts (bone deposition).
• In general, calcitonin has opposite effects to those of parathyroid hormone (PTH). |
|
|
In what vessels would you find the highest concentration of insulin?
|
• Insulin is produced in the beta cells of the pancreatic islets (of Langerhans), as you know.
• Venous blood from the pancreatic islets drains into the hepatic portal vein, via the pancreatic vein, and then into the general circulation. |
|
|
Which zona of the adrenal cortex is the only one capable of synthesizing aldosterone? Why is this true?
|
• Aldosterone is synthesized in the zona glomerulosa alone because this zona is the only one to contain aldosterone synthase.
|
|
|
Which two zonae of the adrenal cortex can synthesize cortisol and corticosterone? Why?
|
• The zona fasciculata and zona reticularis can synthesize the corticosteroids, cortisol and corticosterone, because they contain the necessary enzyme, 17-a-hydroxylase for synthesis.
|
|
|
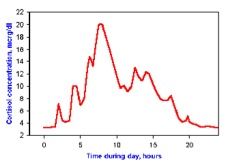
How much daily cortisol is normally secreted from the adrenal glands? How much during stress?
|
• The normal adrenal gland can secrete 20 mg/day of cortisol and may secrete up to 200-500 mg/day during stress. NB: Both the Barash 2009 Handbook and textbook incorrectly state daily cortisol = 200 mg.
• Normal daily 20mg /day cortisol • Stress 200-500 mg/day cortisol |
|
|
..
|
|
|
Perioperative supplemental steroid coverage regimens are either low-dose (physiologic) or high-dose (supraphysiologic). Describe each regimen.
|
• Low-dose (physiologic) supplemental steroid coverage: cortisol 25 mg IV before induction of anesthesia followed by continuous infusion, 100 mg IV over 24 hr.
• Supraphysiologic coverage: cortisol 200-300 mg IV in divided doses on the day of surgery |
|
|
Identify the hallmarks of an acute porphyria attack.
|
• Hallmarks of acute porphyria attacks are abdominal pain, nausea & vomiting, autonomic disturbances with sweating, tachycardia, sustained hypertension, and neurological manifestations including seizures and neuromuscular weakness. Attacks can be life-threatening and neurological manifestations may be permanent.
|
|
|
Describe renal auto regulation. Which renal structure appears to mediate auto regulation?
|
• Renal auto regulation is the mechanism by which the kidney maintains renal blood flow (RBF) and glomerular filtration rate (GFR) thus preserving solute and water regulation independently of wide fluctuations in blood pressure.
• Renal auto regulation typically operates over mean arterial pressures ranging from 60 mm-Hg to 180 mm-Hg (some texts state 80- 180 mm-Hg, and others 60-160 mm-Hg). • Renal vascular resistance appears to be mediated by the variable resistance of the preglomerular afferent arteriole. |
|
|
State and briefly describe two proposed mechanism for renal auto regulation.
|
• The most plausible explanations for renal auto regulations are
› (1) a myogenic response in which the arterioles constrict in response to increased arterial pressure and vice versa, and › (2) tubuloglomerular feedback by way of the juxtaglomerular apparatus. • The myogenic response theory states that increased wall tension in the afferent arterioles, due to an increase in perfusion pressure, causes automatic contraction of the smooth muscle fibers in the vessel wall, thereby increasing resistance to flow, and keeping flow constant despite the increase in perfusion pressure. • The tubuloglomerular feedback mechanism proposes that increased perfusion pressure will increase filtration, increasing the tubular fluid delivery to the macula densa, which then releases a factor or factors that cause vasoconstriction of the afferent arteriole. |
|
|
Identify the 3 major renal processes. Which of these functions require(s) energy (ATP)?
|
• The three major renal tubular functions are filtration, reabsorption, and secretion. Of these, reabsorption and secretion are active transport (ATP) processes that require energy from ATP hydrolysis. The major energy source for reabsorption of sodium, the key link to most renal transport processes, is activity of the Na+--K+ ATPase (pump).
• Remember that "pump" implies an active process, costing ATP-derived energy to move a substance against its concentration gradient. |
|
|
Which nephron tubular segment is responsible for approximately two-thirds of all reabsorption and secretory processes?
|
• Approximately two-thirds of all reabsorption and secretory processes in renal tubules takes place in proximal tubules.
|
|
|
Identify four (4) natriuretic peptides and their sites of synthesis and release in the body.
|
• A series of four similar peptides with natriuretic actions have been identified in the human.
› (1) ANP (atrial or A- type) is released from atrial muscle in response to local wall stretch and increased atrial volume. › (2) BNP (brain or B-type) is released from ventricular muscle when distended. › (3) CNP (C-type) is released from endothelial walls of major vessels. › (4) Urodilatin is produced in the lower urinary tract. |
|
|
Describe the common actions of all natriuretic peptides (ANP, BNP, CNP, urodilatin).
|
• All natriuretic peptides
› induce arterial and venous dilation › increase renal blood flow (RBF) and glomerular filtration rate (GFR) › suppress the actions of norepinephrine, angiotensin, and endothelin. › inhibit renin secretion and decrease angiotensin II-stimulated aldosterone release. › inhibit sodium reabsorption at the collecting duct. |
|
|
Identify the cyclic nucleotide second messenger that mediates the cellular actions of natriuretic peptides.
|
• Cyclic guanosine monophosphate (cGMP) mediates the cellular actions of natriuretic peptides.
|
|
|
State the specificity and sensitivity of the fraction excretion of sodium in distinguishing between prerenal azotemia and acute tubular necrosis (renal azotemia).
|
• The sensitivity and specificity of fractional excretion of sodium of <1% in differentiating prerenal azotemia from acute tubular necrosis are 96% and 95%, respectively.
• Sodium < 1% distinguish prerenal azotemia from ATN › Sensitivity 96% › Specificity 95% |
|
|
What is the most important determinant of blood viscosity?
|
• The most important determinant of blood viscosity is the hematocrit.
• A decrease in hematocrit decreases viscosity and can improve blood flow. • However, there is a concomitant decrease in oxygen-carrying capacity with decreased hematocrit and eventually impaired oxygen delivery. |
|
|
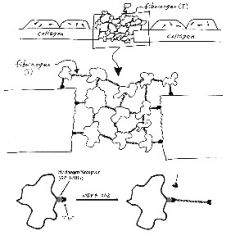
What is GPIIb/IIIa?
|
• GPIIb/IIIa is platelet glycoprotein IIb/IIIa, otherwise known as platelet fibrinogen receptor.
• Upon activation, GPIIb/IIIa binds circulating fibrinogen and promotes platelet aggregation, leading to a primary platelet plug, or white thrombus. |
|
|
..
|
|
|
Identify 4 general indications to administer fresh frozen plasma (FFP).
|
• Transfusions of fresh frozen plasma (FFP) are indicated for:
› (1) isolated coagulation factor deficiencies › (2) reversal of warfarin therapy › (3) correction of coagulopathies associated with liver disease › (4) after massive transfusions with continued bleeding even after platelet transfusions. |
|
|
Describe Virchow's triad.
|
• Virchow's triad is a group of three primary abnormalities in hemostasis that lead to thrombus formation.
• Virchow's triad is › (1) endothelial injury › (2) stasis or turbulent blood flow › (3) hypercoagulability of the blood |
|
|
What type of muscle is the pyloric sphincter?
|
• The pyloric sphincter is a short, relative poor barrier of smooth muscle between the stomach and the duodenum.
|
|
|
What type of muscle is found in the gastrointestinal tract? Identify the arrangement of this muscle in the GI tract.
|
• The tunica muscularis of the gastrointestinal tract is comprised of two layers of smooth muscle: the inner layer is circular, the outer layer is longitudinal.
• Specialized circular muscles are called sphincters. |
|
|
List the electrolyte disturbances associated with anorexia nervosa.
|
• Anorexia nervosa is characterized by hypokalemia, hyponatremia, hypochloremia, and metabolic alkalosis as a result of vomiting and laxative & diuretic abuse.
|
|
|
Identify the two types of acquired immunity.
|
• Acquired immunity is the result of lymphocyte activity and is classified into
› humoral › cell-mediated |
|
|
What specific lymphocyte class mediates humoral immunity? Cell-mediated immunity?
|
• Humoral immunity is mediated by B lymphocytes whereas T lymphocytes are responsible for cell-mediated immunity.
• B lymphocytes get their name from the original discovery of these cells in the bursa of birds. • T lymphocytes are so-named due to their pre-processing and maturation in the thymus gland. |
|
|
List two types of mature B lymphocytes and 4 types of mature T lymphocytes.
|
• Differentiated (mature) B lymphocytes may be either
› memory cells or › plasma cells. • Mature T lymphocytes are either › T-helper (CD4), › T-suppressor (CD8), › T-cytotoxic/killer (CD8), or › T-memory cells. |
|
|
Which lymphocytes are the source of immunoglobulins (Ig)?
|
• Mature B lymphocyte plasma cells are the source of the gamma globulins known as immunoglobulins.
|
|
|
List the 5 types of immunoglobulins in the serum. Approximately what percentage of total serum proteins are immunoglobulins?
|
• The 5 classes of immunoglobulins are IgG, IgA, IgM, IgD, and IgE, collectively accounting for approximately 20% of total serum proteins.
• G-A-M-E-D |
|
|
During an aorto-femoral bypass, the patient becomes hypothermic; what cardiac arrhythmia is likely?
|
• Hypothermia prolongs the refractory period of excitable tissues. In the heart, a prolonged refractory period leads to sinus bradycardia and conduction deficits, which may progress to atrioventricular block and eventually ventricular fibrillation.
|
|
|
Describe the pulmonary mechanics alterations that occur in the patient with end stage scoliosis.
|
• The main alterations in lung mechanics of the patient with end-stage scoliosis are reduced lung volumes (VC, TLC, FRC, and RV-all restrictive process features) and reduced chest wall compliance.
• In the late stages of scoliosis, V/Q mismatching with hypoxia (due to alveolar hypoventilation), increased PAP, hypercapnia, abnormal ventilatory CO2 response curve, increased work of breathing, and cor pulmonale occur, eventually leading to cardiorespiratory failure. |
|
|
The patient is scheduled for lung resection. Identify five findings that would reflect "low risk" postoperative disability.
|
• A "low risk" of postoperative disability following lung resection is predicted when:
› (1) FEVI> 2 L › (2) MVV > 50% predicted › (3) predicted postoperative FEV1> 0.8 L and is 40% of predicted value › (4) RV/TLC <50% › (5) cardiac disease is absent |
|
|
What is a dose-response curve?
|
• A dose-response curve depicts the relationship between the dose of a drug administered (x-axis) and the resulting pharmacologic effect (y-axis).
|
|
|
List four (4) descriptive characteristics of a dose-response curve.
|
• Dose-response curves are characterized by differences in
› (1) potency › (2) slope › (3) efficacy › (4) individual variability. • The potency of a drug is depicted by its location along the dose axis (usually the x-axis) of the dose-response curve. Increased affinity of a drug for its receptor shifts the curve to the left, whereas decreased affinity shifts the curve to the right. • Drug potency and receptor affinity are directly related--a more potent drug has a greater affinity for its receptor. Mnemonic: Left-shift = Less drug required = More potent. |
|
|
What does the slope of a dose-response curve reveal about the drug?
|
• The slope of the dose-response curve indicates the number of receptors that must be occupied (bound) before a drug effect occurs.
• A steep dose response curve slope means that a majority of the receptors must be bound before a relevant effect occurs. • Neuromuscular blocking drugs and inhaled anesthetics dose-response curves have steep slopes. |
|
|
Define drug efficacy. Which feature of a dose-response curve indicates the efficacy of a drug?
|
• Efficacy is a measure of the intrinsic ability of a drug to produce a given physiologic or clinical effect. In other words, the maximal effect of a drug reflects its intrinsic activity, or efficacy.
• A drug's efficacy is depicted by the plateau of the dose-response curve. A higher plateau correlates with a greater efficacy. |
|
|
Describe how the presence of a competitive antagonist would alter a dose-response curve of a drug.
|
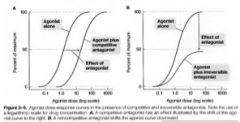
The presence of a competitive antagonist (inhibitor) would shift the dose response curve to the right, with no change in the efficacy (plateau) or slope. The rightward shift is caused by competition for the same number of receptors.
|
|
|
Describe how the presence of a noncompetitive antagonist would alter the dose response curve of a drug.
|

• The presence of a noncompetitive antagonist (inhibitor) would shift the curve rightward and downward, with a decrease in the slope of the curve. The changes occur because a maximal effect cannot be achieved in the presence of a noncompetitive block. In other words, a noncompetitive block cannot be reversed by excess agonist.
|
|
|
What is the "context-sensitive half-time" of a drug and how does it differ from elimination half-time?
|
• The Context-sensitive half-time describes the time necessary for the plasma drug concentration to decrease by 50% (or any other percent) after discontinuing a continuous infusion of a specific duration.
• "Context" refers to the infusion duration. • Context-sensitive half-time, in contrast to elimination half-time, considers the combined effects of distribution and metabolism as well as duration of continuous IV administration on drug pharmacokinetics. |
|
|
What is a substantial limitation to the context-sensitive half-time concept?
|
• The context-sensitive half-time describes only the time to a 50% decrease in the central compartment concentration, which may not correlate with the time to achieve recovery (awaken, for example).
|
|
|
Give the chemical formula for each of the clinically useful inhaled anesthetic agents. For example nitrous oxide is N20.
|
• Nitrous oxide: N20
• Halothane: C2HBrClF3 • Enflurane: C3H2C1F50 • Isoflurane: C3H2ClF50 • Desflurane: C3H2F60 • Sevoflurane: C4H3F70 |
|
|
Write out the condensed structural formula for each of the clinically useful inhaled anesthetic agents. For example, nitrous oxide is N=N-0.
|
• Nitrous oxide: N=N-0
• Halothane: CF3CHBrCI • Enflurane: -CHF2-0-CF2-CHCIF • Isoflurane: -CHF2-0-CHCl-CF3 • Desflurane: -CHF2-0-CHF-CF3 • Sevoflurane: -CH2F-O-CH-(CF3)2 |
|
|
State the trade names of each of the volatile inhaled agents.
|
• Halothane is Fluothane
• enflurane is Ethrane • isoflurane is Forane • desflurane is Suprane • sevoflurane is Ultane |
|
|
Give the IUPAC (International Union of Applied and Applied Chemistry) chemical names of each of the clinically useful inhaled names of each of the clinically useful inhaled names of each of the clinically useful inhaled anesthetic agents.
|
• Nitrous oxide: dinitrogen monoxide.
• Halothane: 2-bromo-2-chloro-1,1,1-trifluroethane • Enflurane: 2-chloro-1,1,2,-trifluoroethydli fluoromethyl ether • Isoflurane: 1-chloro-2,2,2-trifluoroethydli fluoromethyl ether • Desflurane: difluoromethyl-1-fluoro-2,2,2 trifluoroethyl ether • Sevoflurane: fluoromethyl-2,2,2-trifluoro-1 [trifluoromethyl] ethyl ether. |
|
|
Enflurane, isoflurane, desflurane and sevoflurane all have a "C-0-C" bond their structures. What is the name of the C-0-C functional group?
|
• The functional group C-0-C is called an ether bond. A functional group is a set of atoms bonded together in a specific way.
• C-O-C = ETHER |
|
|
Which of the clinically useful volatile inhaled agents are ethers?
|
• All of the volatile agents except halothane are ethers
• Halothane IS NOT an ether |
|
|
Halothane is not an ether; from what functional group is halothane derived?
|
• Halothane is a halogenated alkane derivative. An alkane is a hydrocarbon containing all carbon-carbon single bonds (no double or triple bonds between carbon atoms).
• Halothane is HALOGENATED ALKANE |
|
|
Which three modern volatile agents are methyl ethyl ethers?
|
• Enflurane, isoflurane, and desflurane are methyl ethyl ethers.
|
|
|
If sevoflurane is not a methyl ethyl ether, what type of ether derivative is sevoflurane?
|
• Sevoflurane is fluorinated methyl isopropyl ether.
|
|
|
Which two clinically useful volatile inhaled agents are isomers of each other? (Hint: recall that isomers have identical chemical formulas but different arrangements of the atoms...)
|
• Enflurane and isoflurane are isomers of each other (the "iso" in isoflurane is a tip-off)
|
|
|
Which of the modern volatile anesthetic is the only one containing a bromine
|
• Halothane is the only brominated modern volatile anesthetic agent
|
|
|
Which two modern volatile agents have - only fluorine as the halogen substitutions?
|
• Desflurane and sevoflurane are halogenated exclusively with fluorine.
• Volatile agents halogenated exclusively with fluorine are less soluble in blood. |
|
|
Which modem volatile agent differs from isoflurane only in the replacement of the chlorine with fluorine?
|
• Desflurane differs from isoflurane only by the substitution of a fluorine atom for the chlorine atom.
• This one change increases the vapor pressure dramatically (669 mm-Hg for desflurane compared to 240 mm-Hg for isoflurane). |
|
|
List four contraindications to the use of N2O.
|
• (1) Presence of a closed pneumothorax
• (2) tympanoplasty or other middle ear surgery • (3) pneumocephalus • (4) venous air embolism |
|
|
Which volatile agents most depress the baroreceptor reflex, and which least depress it?
|
• Halothane and sevoflurane most depress the baroreceptor reflex (there are no increases in heart rate despite decreases in blood pressure with these agents). Depression of the baroreceptor response by sevoflurane is comparable to halothane.
• In contrast, isoflurane and desflurane least depress the baroreceptor reflex (heart rate tends to increase reflexively with the decreases in blood pressure produced by these agents). |

