![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
6 Cards in this Set
- Front
- Back

|
The Greeks(450 to 350 B.C.E., Before Common Era) The ancient Greek people believed that all matter was made of the four “elements”: fire, air, earth & water. |
|

|
John Dalton,(1800s)An English School teacher and a Chemist. suggested that all matter is made up of Particles could not be divided. Dalton believed that each element has its own unique properties. When these atoms combine, they make new substances. (Couldn’t be divided, billiard ball model) |
|

|
William Crookes and J.J. Thomson (late 1800s) William Crookes invented the cathode ray tube. He also created the “Plum Pudding Model” stating that there are both positive and negative electrons.
|
|
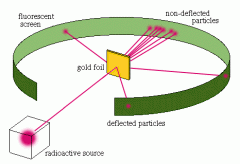
|
Rutherford’s Gold Foil Experiment (1911)Rutherford said that atoms contain a tiny, but very massive nucleus, made up of positively charged protons, surrounded by a cloud of electrons. |
|

|
Bohr and Flame Tests(1912)Niels Bohr studied the element hydrogen. When he added energy, the atoms always gave off the same 4 colors of light. Each element has its own line spectrum (like a lightly fingerprint) of light. |
|
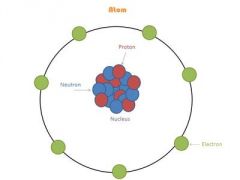
|
Chadwick and the Neutron (1932)Third particle in the atom was found, called the neutron. the nucleus and has no electrical (it’s neutral)
|

