![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
metabolic pathway |
aspecific molecule and ends with a product |
|
|
catalyst |
achemical agent that speeds up a reaction without being consumed by the reaction |
|
|
enzyme |
acatalytic protein |
|
|
enzyme-catalyzed reaction |
Hydrolysis of sucrose by the enzymesucrase isan example of |
|
|
a slow process |
The reaction sucrose --> glucose +fructose is normally |
|
|
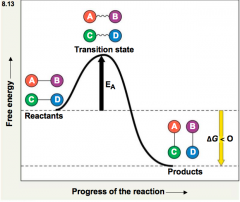
activation energy |
Theinitial energy needed to start a chemical reaction -oftensupplied in the form of thermal energy that the reactant molecules absorb fromtheir surroundings -whenthe reactant molecules have absorbed enough energy, the bonds become unstable andthe molecule releases the transition state. |
|
|
exergonic |
|
|
|
lowering the Ea threshold; usinghigh temperature would denature proteins and kill cells or speed up all reactions withinthe cell. |
Enzymescatalyze reactions by what instead of using high temps |
|
|
substrate |
Thereactant that an enzyme acts on is calledthe enzyme’s |
|
|
enzyme-substrate complex |
Theenzyme binds to its substrate, forming an |
|
|
active site |
the region on the enzymewhere the substrate binds; not rigid changes to fit enzyme |
|
|
induced fit |
a substrate brings chemical groupsof the active site into positions that enhance their ability to catalyze thereaction |
|
|
-Orienting substrates correctly -Straining substrate bonds -Providing a favorable microenvironment -Covalently bonding to the substrate (avery brief covalent bond is formed that is removed by subsequent reactions) |
Theactive site can lower an Ea barrier by: |
|
|
-General environmental factors, such astemperature and pH -Chemical influence |
enzyme’sactivity can be affected by |
|
|
-Substrate concentration -enzyme concentration |
The speed of enzyme activity (how efficient it functions) canbe influenced by |
|
|
-pH (human normal pH 6-8) -temperature (35-40C) |
enzymes have optimal |
|
|
cofactors |
non-proteinenzyme helpers |
|
|
-inorganic ex. zinc, copper -organic (coenzyme) ex. vitamins |
Cofactorsmay be |
|
|
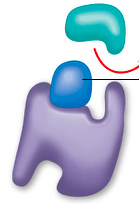
competitive inhibitors |
bindto the active siteof an enzyme, competing with the substrate ex. penicillin, sarin nerve gas, and methanol |
|
|
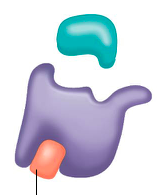
noncompetitive inhibitors |
bindto another part of an enzyme(NOT the active site), causing the enzyme to change shapeand making the active site less effective ex. heavy metal poisoning |
|
|
competitive inhibition |
|
|
|
noncompetitive inhibition |
|
|
|
allosteric regulation |
aregulatory molecule binds to a protein at one site andaffects the protein’s function at another site -mayeither inhibit or stimulate an enzyme’s activity |
|
|
activator |
Thebinding of an ______ stabilizes the active form of theenzyme |
|
|
inhibitor |
Thebinding of an ______ stabilizes the inactive form ofthe enzyme |
|
|
cooperativity ex. hemoglobin |
A form of allosteric regulationthat can amplify enzyme activity -onesubstrate molecule primes an enzyme to act on additional substrate moleculesmore readily - allostericbecause binding by a substrate to one active site affects catalysis in adifferent active site |
|
|
feedback inhibition ex. isoleucine synthesis; enzyme threonine |
theend product of a metabolic pathway shuts down the pathway - preventsa cell from wasting chemical resources by synthesizing more product than isneeded |

