![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
89 Cards in this Set
- Front
- Back
|
Karl Scheele |
Founder of Biochemistry |
|
|
Karl Scheele |
Studied the composition of matter in 1700 |
|
|
Schleiden and Schwann |
Formulated the Cell Theory in 1840 |
|
|
Walther Flemming |
Discovered chromosomes in 1875 |
|
|
Carl Newberg |
German scientist who coined the word 'Biochemistry' |
|
|
Hans Kreb |
Proposed the Kreb Cycle in 1937 |
|
|
Embden & Meyerhoff |
Described the glycotic pathway in 1925 |
|
|
James Watson & Francis Crick |
Described the double helical structure of DNA |
|
|
Edward & Hans Buchner |
Found out that extracts from yeasts could bring about the fermentation of sugar into alcohol in 1897 |
|
|
Paul Boyer and J. Walker |
Discovered the "Rotary Engine" that generated ATP in 1997 |
|
|
Danish J. Skou |
Studied the "pump" that drives sodium and potassium across membranes |
|
|
Stanley Prusiner |
Discovered the organism that caused "mad cow disease" |
|
|
Ruska, et. al. |
Discovered the electron microscope |
|
|
Structural Metabolism Genetics |
3 areas of Biochemistry |
|
|
Biomolecules |
Molecules found in living matter |
|
|
Small molecules Macromolecules |
Two broad types of biomolecules |
|
|
Amino Acids |
Monomers of proteins |
|
|
Nitrogenous bases |
Pyramidines and Purines |
|
|
Sugars |
Glucose, galactose, mannose |
|
|
Sugar alcohol |
Example: Glycerol |
|
|
Nitrogenous alcohol |
Example: Choline |
|
|
Fatty Acids |
Examples: almitic acid, linoleic acid, arachidonic acid |
|
|
H+ and OH- |
Ionization products of water |
|
|
Hydrogen bonding |
Enables water to dissovle many organic biomolecules |
|
|
H-bonds |
Account for the surface tension, viscosity, liquid state at room temperature, and solvent power of water. |
|
|
high H+ conc |
Low pH values |
|
|
low H+ conc |
High pH values |
|
|
Acids |
Proton donors |
|
|
Bases |
Proton acceptors |
|
|
Hydrogen bonding |
Enables water to dissolve many organic biomolecules that contain functional groups |
|
|
Oxidation |
Process wherein most of the energy liberated by living matter is derived from the oxidation of organic substances such as carbohydrates, fats, and proteins. |
|
|
Reduction |
Loss of oxygen or by gain of hydrogen or electrons. |
|
|
Hydrolysis |
Union of a substance with one or more molecules of water. |
|
|
Hydrolysis |
Large molecules are broken down into smaller and simpler forms. |
|
|
Condensation |
Simple fragments unite with one another to form a more complex compound. |
|
|
Tautomerism |
Also known as isometric transformation. |
|
|
Tautomerism |
Intramolecular rearrangement of atoms within a molecule. |
|
|
Diffusion |
Interpenetration of molecules between two substances. |
|
|
Diffusion |
Occurs whenever the solute distributes itself uniformly into the solvent. |
|
|
size of molecules, temperature, molecular weight |
factors affecting diffusion |
|
|
Osmosis |
Passage of water molecules from high to low concentration or from high osmotic pressure to low osmotic pressure through a semi-permeable membrane. |
|
|
Isotonic |
Equal concentration of ions in solution and cell |
|
|
Hypertonic |
High concentration of ions vs the cell |
|
|
Hypotonic |
Low concentration of ions in solution vs the cell |
|
|
Nucleotides Amino Acids Monosaccharides Fatty Acids |
Building Blocks |
|
|
Precursors from the Environment Metabolic Intermediates Building Blocks Macromolecules Supramolecular Assemblies Organelles |
Hierarchy in the Molecular Organization of Cells |
|
|
Nucleic Acids Proteins Polysaccharides Lipids |
Macromolecules |
|
|
Nucleus Mitochondria Golgi Complex Endoplasmic Reticulum Lysosomes |
Organelles |
|
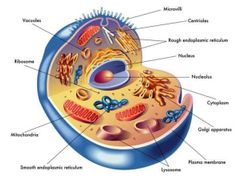
|
Parts of the Cell |
|
|
Nucleus |
Contains most of the cell's genes and is usually thr most conspicuous organelle |
|
|
Nuclear Envelope |
Encloses the nucleus, separating it from the cytoplasm. |
|
|
Nuclear Lamina |
Maintains the shape of the nucleus and is composed of proteins |
|
|
Chromosomes |
In the nucleus, the DNA is organized into discrete units called? |
|
|
Chromatin |
The DNA and proteins of chromosomes together |
|
|
Chromatin |
Condenses to form discrete chromosomes as a cell prepares to divide |
|
|
Nucleolus |
Located within the nucleus and is the site of ribosomal RNA (rRNA) synthesis |
|
|
Ribosomes |
Complexes of rRNA and protein |
|
|
Endoplasmic Reticulum (ER) |
Accounts for more than half of the total membrane in many eukaryotic cells. |
|
|
Smooth ER Rough ER |
Two distinct regions of ER |
|
|
Smooth ER |
lacks ribosome |
|
|
Rough ER |
Studded with ribosomes |
|
|
Functional Groups |
The basis for the classification of organic compounds into families |
|
|
Alkanes |
Simplest family of molecules |
|
|
Straight-chain alkane |
Carbon connected in a row; normal alkanes |
|
|
Branched-chain alkanes |
Examples: 2-methylpropane, 2,2-dimethylpropane |
|
|
Alkyl Groups |
Removing a hydrogen atom from an alkane |
|
|
Dichloromethane |
Widely used haloalkane solvent |
|
|
Alkenes and Alkynes |
Unsaturated hydrocarbon that contains a carbon-carbon double bond. |
|
|
120 degrees |
Bond angle for alkenes and alkynes |
|
|
Rhodopsin |
Reddish compound |
|
|
Terpenes |
The essential oil found in plants |
|
|
Benzene |
Six carbon rings with alternating single and double bonds and one hydrogen bonded to each carbon |
|
|
Contributing structure |
Each Lewis structure is called a |
|
|
Phenyl Group |
The aryl group derived by removing a hydrogen atom from benzene. |
|
|
Arenes |
Alkyl-substituted benzenes |
|
|
Carcinogen |
A compound that causes cancer |
|
|
Polynuclear Aromatic Hydrocarbons (PAHs) |
Contains two or more benzene rings |
|
|
Thyroxine |
Hormone produced in the thyroid glands |
|
|
Trinitrololuene |
TNT; explosive |
|
|
Ketone |
Carbonyl is bonded to 2 carbon atoms |
|
|
Acetone (propanone) |
Simplest ketone |
|
|
Aldehyde |
Carbonyl is bonded to a hydrogen atom |
|
|
Methanal |
Simplest aldehyde |
|
|
Aliphatic Amine Aromatic Amine Heterocyclic Amine Heterocyclic Aliphatic Amine Heterocyclic Aromatic Amine |
Types of Amines |
|
|
Aliphatic amine |
Type of amine where all the carbons bonded to nitrogen is derived from alkyl groups |
|
|
Aromatic amine |
One or more of the groups bonded to nitrogen are aryl groups |
|
|
Heterocyclic amine |
An amine in which nitrogen is one of the atoms of a ring |
|
|
Heterocyclic aliphatic amine |
When the ring is saturated |
|
|
Heterocyclic aromatic amine |
An amine in which nitrogen is one of the atoms of an aromatic ring; purines and pyramidines |

