![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
299 Cards in this Set
- Front
- Back
|
physiologic changes that occur during pregnancy
|
-anatomy and physiology change as a result of altered hormonal activity
-biochemical changes occur which are associated with increasing metabolic demands of the growing fetis, placenta, and uterus -mechanical displacement occurs by the enlarging uterus |
|
|
what are women at an increased risk for beyond midgestation?
|
-for pulmonary aspiration of acidic gastric contents because of decreased competence of the lower esophageal sphincter, and due to delayed gastric emptying seen with the onset of labor or use of opioids
|
|
|
summary of cardiovascular changes during pregnancy
|
1. an increase in intravascular volume
2. an increase in CO 3. a decrease in SVR |
|
|
effects of pregnancy on maternal intravascular volume
|
-maternal fluid volume starts to increase in the first trimester, and by term it has increased by about 45%
|
|
|
what happens to maternal erythrocyte volume?
|
it increases by 20%
|
|
|
what does this mean?
|
it means that the larger increase in maternal intravascular fluid volume compared to the increase in maternal erythrocyte volume accounts for the relative anemia of pregnancy
|
|
|
why does intravascular volume increase in pregnancy?
|
the extra volume helps offset the 300-500ml loss of blood lost during vaginal delivery, and the 800-1000ml during C-section
|
|
|
total plasma protein concentration during pregnancy
|
is decrease as a result of dilution secondary to the increased volume
|
|
|
changes in CO during preganncy
|
-by the 10th week of gestation its increased by 10%, and by term its increased 40-50%
|
|
|
how is the CO augmented in the pregnant patient?
|
by a 25-30% increase in SV, and a 15-25% increase in HR
|
|
|
when else does CO change in these patients?
|
with the onset of labor
|
|
|
CO during labor
|
-it starts to increase after labor, with the largest increase occuring right after delivery, when CO is uo 80%
|
|
|
consequences of this increase in CO
|
-presents a postpartum risk in patients with cardiac disease, like fixed valvular stenosis
|
|
|
what can be done to attenuate the maternal tachycardia and hypertension in these patients?
|
a regional anesthetic can stop the release of catecholamines
|
|
|
return of CO to normal
|
returns substantially to prepregnancy levels by 2 weeks postpartum
|
|
|
systemic blood pressure and pregnancy
|
-although there is an increase in plasma volume and CO, systolic BP decreases by 15% during prenancy as a result of decreased SVR
|
|
|
what about mean arterial pressure?
|
there may be a slight decrease
|
|
|
what about central venous pressure?
|
-despite the increase plasma volume, there is no change in CVP during preganncy, because there is increased venous capacitance
|
|
|
where is venous pressure increased?
|
femoral venous pressure increases by 15% or so, as a result of IVC compression by the gravid uterus
|
|
|
in the pregnant patient, what are decreases in maternal blood pressure associated with?
|
the supine position, as the gravid uterus compresses the aorta and cava
|
|
|
mechanism of supine hypotension syndrome
|
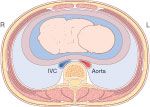
-the decreased venous return that results from compression of the IVC by the gravid uterus leads to a decrease in CO and decline in systemic BP
|
|
|
compensation for the decreased venous return
|
-one mechanism is increase dvenous pressure below the level of IVC compression, which diverts blood from the lower half of the body through the paravertebral plexus to the azygous vein, which enters the SVC maintaining venous return
-a second is a reflex increase in peripheral sympathetic activity, causing increased SVR an maintenance of BP despite decreased CO |
|
|
what implication does the first of these compensatory mechanisms have?
|
-the epidural veins are dilated, making venous penetration during lumbar epidural placement more likely, which could lead to accidental injection of local intravscularly, causing the profound CNS and cardiac effects
|
|
|
what is important to remember about the second of the compensatory effects?
|
-that the compensatory increases in SVR will be impaired by regional anesthesia
-it has indeed been shown that arterial hypotension during regional is more profound and common in pregnant than nonpregnant patients |
|
|
arterial compression by the gravid uterus
|
-leads to arterial hypotension in the lower extremities, but maternal symptoms are usually not seen, and decreases in systemic blood pressure measured in the arms wont be seen
|
|
|
what does vena caval compression result in?
|
it causes lower extremity venous stasis, which results in lower extremity edema and varices
|
|
|
supine hypotension syndrome
|
-when these changes in the supine pregannt patient cause the symptoms of diaphoresis, nausea, vomiting, and changes in cerebration
|
|
|
what is the significant risk of aortocaval compression?
|
-the associated decrease in uterine and placental blood flow
-even with a healthy uteroplacental unit prolonged maternal hypotension (90-100 mmHg for more than 10-15 min) is likely to significantly decrease uterine blood flow and cause progressive fetal acidosis |
|
|
what does vena caval compression result in?
|
it causes lower extremity venous stasis, which results in lower extremity edema and varices
|
|
|
supine hypotension syndrome
|
-when these changes in the supine pregannt patient cause the symptoms of diaphoresis, nausea, vomiting, and changes in cerebration
|
|
|
what is the significant risk of aortocaval compression?
|
-the associated decrease in uterine and placental blood flow
-even with a healthy uteroplacental unit prolonged maternal hypotension (90-100 mmHg for more than 10-15 min) is likely to significantly decrease uterine blood flow and cause progressive fetal acidosis |
|
|
treatment of aortocaval compression
|
-want to displace the gravid uterus, especially in patients having regional or general anesthesia which will blunt the compensaotry mechanisms
-can do this by placing the patient lateral, or moving the uterus to the left off of the IVC and aorta |
|
|
displacing the uterus to the left
|
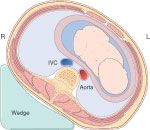
-can be done by elevating the right hip 10-15 cm with a blanket or wedge
|
|
|
overview of pulmonary changes seen in pregnancy
|
changes in:
1. the upper airway 2. minute ventilation 3. lung volumes 4. arterial oxygenation |
|
|
upper airway chnages during preganncy
|
-get capillary engorgement of the mucosal lining of the upper respiratory tract
|
|
|
implications of this
|
-need to be careful when instrumenting the upper airway (avoid nasal instrumentation if possible), during suctioning, airway placement, and direct laryngoscopy
-may need to use smaller cuffed tracheal tubes (6.5-7.0) sinc ethe vocal cords and arytenoids are often edematous |
|
|
what is the implication of weight gain during pregnancy, especially in patients with coexisting obesity or short stature?
|
these can result in difficulty inserting the laryngoscope due to a short neck and large breasts
|
|
|
changes in minute ventilation during pregnancy
|
-increases about 50% in the first trimester, and stays that way throughtout
|
|
|
how is this increase in minute ventilation achieved?
|
-primarily by an increase in tidal volume (about 40%)
-versus a relatively small increase in rate (15%) |
|
|
why is minute ventilation thought to increase?
|
because of the increase in progesterone
|
|
|
what does this change in minute ventilation change?
|
the PaCO2
|
|
|
what happens to the PaCO2?
|
the resting PaCO2 decreases from 40mmHg to 32 mmHg during the first trimester
|
|
|
what happens to the pH as a result?
|
-it does not change, since the kidneys simply excrete more bicarbonate
|
|
|
what will then happen to ventilation during labor?
|
-hyperventilation will get worse during labor due to the pain (though this is attenuated with adequate analgesia)
|
|
|
lung volume changes during pregnancy
|
-even though minute ventilation starts to increase in the first trimester, lung volumes dont start to change until the third month of pregnancy
-as the uterus engorges the diaphragm is pushed cephalad, causing about a 20% decrease in FRC (and RV and ERV) |
|
|
what is the result in this decrease in FRC?
|
the FRC can become less than the closing capacity of the small airways, causing atelectasis in the supine position
|
|
|
how does vital capacity change?
|
it doesnt really
|
|
|
what does the combination of increased minute ventilation and decreased FRC result in?
|
-an increased rate at which changes in inhaled anesthetic alveolar concentrations occurs, affecting induction, emergence, and change in depth of anesthesia
|
|
|
changes in arterial oxygenation during pregnancy
|
-early in gestation while breathing room air, maternal paO2 will be >100mmHg, reflecting the hyperventilation
-later, though, it will become normal or a little decreased likely due to airway closure |
|
|
what things should be kept in mind during induction in pregnant patients in terms of ventilation?
|
-due to the decreased O2 reserve (dec FRC) and the increased O2 uptake (inc metabolic rate) desaturation will be more rapid, so supplemental oxygen should be given during regional, and preoxygenation should be done before any anticipated periods of apnea
|
|
|
anesthetic requirements in pregnancy
|
-the MAC for volatile agents is decreased, likely due to the sedating effect of progesterone
|
|
|
clinical implications of the decreased MAC
|
-alveolar concentrations of the inhlaed agents that wouldnt produce unconsciousness in nonpregnant patients may be enough for anesthesia
-and keep in mind that this degree of anesthesia can impair protective upper airway reflexes and risk aspiration |
|
|
other important point to remember in terms of inhaled anesthetics
|
as mentioned before, the decreased FRC increases the speed at which you can get excessive alveolar concentrations of the agents
|
|
|
regional anesthetic changes during pregnancy
|
-get engorgement of the epidural veins due to the increased intraabdominal pressure from the enlarging uterus, which causes a decrease in size of the epidural space and decrease in the volume of CSF
|
|
|
what is the implication of the decreased size of the space and volume of CSF?
|
-it facilitates the spread of local anesthetic
-indeed the increased spread of local in the epidural space is seen as early as the first trimester, which indicates it isnt just the mechanical changes which causes this but also biochemical changes -also, there are decreased dose requirements of local for epidurals and spinals in term patients -pregnant patients are also shown to have increased peripheral nerve sensitivity to lidocaine |
|
|
renal changes seen in pregnancy
|
-renal blood flow and GFR increase by 50-60% by the 3rd month of pregnancy
|
|
|
why is this important?
|
so you know that the upper limit of normal for BUN and Cr is decreased by 50% in pregnant patients
|
|
|
hepatic changes seen in pregnancy
|
-plasma protein concentrations will be reduced (similar to the reason for anemia)
|
|
|
what is the implication of this?
|
-there will be a decrease in serum albumin levels, which causes a higher level of free drug for highly-protein bound drugs
-also, plasma cholinesterase levels 25% from the 10th week until as far as 6 weeks postpartum |
|
|
what is the implication of the decrease in pseudocholinesterase?
|
-it is not likely to prolong to action of succ or mivacurium
|
|
|
circulating coagulation factor levels during pregnancy
|
-coagulation factor levels, including of fibrinogen, will be increased, concistent with the hypercoagulable state of pregnancy
|
|
|
what does it mean if LFTs are slightly elevated during pregnancy?
|
doesnt necessarily mean hepatic disease
|
|
|
GI changes in pregnancy
|
women are at increase drisk of aspiration of gastric contents, and those contents causing acid pneumonitis, due to several reasons:
-the pylorus is displaced cephalad, which retards gastric emptying -progesterone decreases gastric motility, so gastric fluid volume is increased, even in the fasting state -the placenta secretes gastrin, which stimulates stomach cells to make H+ ions, making the pH lower -the uterus changes the angle of the GE junction, causing incompetence of the LES (this is why reflex and esophagitis are common in the pregnant) |
|
|
preventing aspiration in pregnant patients
|
-no matter when they last ate, consider them to have a full stomach, esp since pain, anxiety, and drugs (esp opioids) can even further slow stomach emptying
-always use a cuffed tube -since the pH of gastric contents is the important factor in causing pneumonitis, might be wise to give nonparticulate antacids to pregnant patients before induction, such as sodium citrate |
|
|
pretreating stomach acidity before induction
|
-in addition to nonparticulate antacids, H2 blockers increase the pH of gastric fluid, without many side effects
-they dont change the pH of gastric fluid already in the stomach though, so it might be best to combine them with the antacid, which does |
|
|
another thing you can do in an at risk for aspiration patient?
|
-can use metoclopramide to increase gastric motility and decrease volume, esp in high risk patients such as those who have recently eaten, are apprehensive, or are on opioids
-the problem is patients who have gastric hypomotility due to opioids might be resistant to metoclopramide treatment |
|
|
the placenta and its blood flow
|
-the placenta is a union of fetal and maternal tissue whose purpose is physiologic exchange between the two
-maternal blood is delivered to it by the uterine arteries -fetal blood is delivered to it by 2 unbilical arteries -nutrient rich waste-free blood is delivered to the fetus by 1 umbilical vein |
|
|
normal uterine blood flow during pregnancy
|
-it increases to about 700 ml/min (10% of CO) by term, with 80% of this blood going to the intervillous space of the placenta, and 20% to the myometrium
|
|
|
regulation of the uterine vasculatrue
|
-it is not autoregulated, and will basically stay maximally dilated under normal circumstances
-it can, though, be constricted by a-adregnergic drugs, though pregnancy is known to be associated with decreased response and sensitivity to these by the uterine vasculature |
|
|
so what will cause uterine blood flow to decrease?
|
-a decrease in perfusion pressure due to systemic hypotension (ie. shock, spinal/epidural/general anesthesia)
-also due to aortocaval compression, which caused increased uterine venous pressure -also, uterine contractions (especially with hyperstimulation by oxytocin, or abruption) |
|
|
effect of epidural or spinal on uterine blood flow
|
does not alter it, as long as hypotension is successfully avoided
|
|
|
treatment of hypotension in pregnant patients
|
-it was shown in animals that alpha stimulation caused increased uterine vascular resistance and decreased uterine blood flow, whereas ephedrine increased arterial blood pressure without these changes
-the applicability of this to humans has been questioned, as using alpha agonists to treat hypotension has not been shown to have negative effects on fetal well-being -so thereofre, ephedrine is usually the drug of choice, though phenylephrine is more widely being used also |
|
|
ephedrine effects when compared to phenylephrine
|
ephedrine has been shown to cause clinically insignificant umbilical cord blood acidemia compared to phenylephrine
|
|
|
so final say in treating maternal hypotension
|
-caution should be shown in patients who already have known uteroplacental insufficiency, as maternal hypotension and its correction with alpha agonists might further compromise the fetus
-otherwise, either ephedrine or phenylephrine has been used successfully, the most important thing is correcting maternal hypotension will lead to the best fetal outcome |
|
|
what else can cause increased uterine vascular resistance and decreased uterine blood flow?
|
-maternal stress or pain which stimulates catecholamine release
-this suggests a protective effect on the fetus with regional or general anesthetic, as long as hypotension is avoided |
|
|
how does placental exchange of substances occur?
|
-by diffusion from the maternal to fetal circulation, or vice versa
|
|
|
what does diffusion from the mother to fetus across the placenta depend on?
|
-maternal to fetal concentration gradients
-maternal protein binding (the more protein bound the less will cross) -molecular weight (the higher the weight the less will cross) -lipid solubility (the less soluble the less will cross) -and degree of ionization (the more ionized the less will cross) -basically, drugs that readily cross the BBB will the placenta |
|
|
muscle blockers and the placenta
|
-the high molecular weight and low lipid solubility on nondepolarizers means they dont really cross the placenta
-even though succ is low in weight, it is highly ionized so doesnt readily cross the placenta -thus, during general, the fetus is not paralyzed |
|
|
other drugs we use and the placenta
|
-barbiturates, local, and opioids do readily cross the placenta due to their low molecular weights
|
|
|
pH of fetal versus maternal blood
|
-the pH of fetal blood is lower (more acidic by 0.1 unit) than maternal
|
|
|
implication of this pH difference
|
-the lower fetal pH means that weakly basic drugs, such as local and opioids, which cross the placenta in inonionized form will become ionized once in the fetal circulation, and since ionized drugs cant cross the placenta they cant cross back, so they will accumulate in the fetal blood against their concentration gradient
-known as ion trapping |
|
|
drugs once in the fetal circulation
|
-even though the fetus has decreased enzyme activity compared to adults, the fetal enzyme systems are adequate to metabolize most drugs
|
|
|
what is the exception to this?
|
mepivacaine
|
|
|
unique characteristics of the fetal circulation
|
-has features which influence how drugs are distributed and protect the fetus from exposure to high concentrations of drugs in umbilical venous blood
-75% of umbilical venous blood passes through the liver, so a large portion of the drugs are metabolized before reaching the fetal circulationand delivery to the heart and brain -also, drugs in the umbilical venous blood which enters the IVC will be diluted by drug-free blood returning from the lower extremities and pelvic viscera of the fetus |
|
|
things that cause pain during labor and delivery
|
-uterine contractions
-dilation of the cervix -distention of the perineum |
|
|
afferent fibers from the uterus and cervix
|
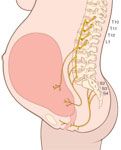
--somatic and visceral afferent sensory fibers from the uterus and cervix travel with sympathetic nerve fibers to the spinal cord; they travel through the paracervical tissue with the uterine artery, then through the inferior, middle, and superior hypogastric plexuses to the sympathetic chain
|
|
|
nerve impulses from the uterus and cervix
|
enter the spinal cord through T10-12 and L1
|
|
|
somatic perineal pain impulses
|
-travel via the pudendal nerve to S2-4
|
|
|
what is pain from the perineum associated with?
|
-its caused by distention of the vagina, perineum, and pelvic floor muscles, and is associated with descent of the fetus into the pelvis during the 2nd stage of labor
|
|
|
somatic vs. visceral pain
|
-somatic pain, which includes incisional pain and 2nd-stage of labor pain, is well-localized and sharp
-visceral pain, such as with uterine contractions in the 1st stage of labor, is poorly localized and "dull but intense aching" |
|
|
along with the sensation of pain, what do the nerve impulses generated by labor pain lead to?
|
-sympathetic stimulation, leading to reflex CV, respiratory, endocrine, and musculoskeletal effects, like tachycardia, hypertension, increased levels of catecholamines, and reduced uterine blood flow
|
|
|
why is analgesia for labor and childbirth important?
|
-it reduces the psychological or subjective component of pain (or both), and may prevent reflex effects which might be deleterious to certain high-risk patients or their fetuses (such as those with preeclampsia, valvular heart disease, myasthenia gravis, etc.)
|
|
|
factors that might influence the perception of labor pain
|
include:
-duration of labor -maternal pelvic anatomy and fetal size -use of oxytocin -parity -participation in childbirth classes -fear and anxiety about childbirth -attitudes and experience of pain -coping mechanisms |
|
|
use of systemic medications for analgesia for labor and delivery
|
-are widely used, but have to be given in limited doses, as they cross the placenta and depress the fetus in a dose-dependent fashion
|
|
|
what is the most commonly used systemic medication for labor and delivery?
|
opioids
|
|
|
problem with opioids
|
-the amount that can bi given safely to a pregnant woman is limited by their potential to cause maternal sedation, maternal respiratory depression, loss of maternal airway reflexes, and the risk of neonatal depression
|
|
|
most commonly used opioids in OB
|
-the synthetic opioids, such as fentanyl, butorphanol, nalbuphine, meperidine; and morphine
|
|
|
in which OB patients are opioids best used?
|
-in primes in early labor
-as adjuncts to regional -in multips with short, predictable labor with minimal pain |
|
|
benzos and obstetrics
|
-diazepam and midazolam are the commonly used ones in OB
-they rapidly cross the placenta and within minutes the levels in fetal and maternal blood are equal -neonates have a limited ability to metabolize diazepam, so it and its metabolites can remain in the system for a week -when use din small doses of 2.5 - 10mg IV see minimal newborn sedation and hypotonia |
|
|
ketamine and OB
|
-can be given IM or IV at low-doses (0.25 mg/kg) to produce dissociative analgesia (characterized by profound analgesia and amnesia without loss of consciousness or protective airway reflexes)
-can be used to produce this during vaginal delivery and episiotomy repair at divided doses totalling 1 mg/kg -is best used in low-doses to supplement other techniques |
|
|
opioids during OB
|
-the dose given is intended to produce analgesia with minimal side effects to the mother or fetus, but the problem is this dose does not provide adequate analgesia for labor and delivery in many women, so their use is declining
-further, all cross the placenta and given fetal effects at normal doses, causing decreased fetal heart rate variability |
|
|
maternal side effects of opioids
|
-nausea and vomiting
-pruritis -decreased GI motility |
|
|
what is the most commonly used technique for labor analgesia?
|
regional (epidural, spinal, CSE)
|
|
|
local anesthetics used for regional in OB
|
-can use either amides or esters: esters are rapidly broken down by plasma cholinesterase limiting the risk of maternal drug transfer and toxicity, whereas amides are slowly degreaded by the liver and bind to plasma proteins
|
|
|
how is a mild overdose of local anesthetic manifested?
|
by the neonate as a decrease in neuromuscular tone similar to that seen with magnesium
|
|
|
what is the analgesic method of choice for labor and delivery?
|
epidural anesthesia, which provides analgesia while the woman remains awake without sedative effects
|
|
|
other advantages of epidural
|
-reduced catecholamine concentrations
-avoiding hyperventilation -the mother is able to cooperate and participate actively in labor |
|
|
further advantages of epidurals during LABOR
|
-have the ability to get segmental bands of analgesia at T10-L1 during the first stage of labor when you dont need total analgesia
-can then extend the block to S2-4 during the 2nd stage -can extend the sensory level to T4 for C-section |
|
|
epidural technique
|
-make sure to have airway and resuscitation equipment available
-go betwene the spinous processes of most commonly L3-4 or L4-5 until there is loss of resistance to air or saline, then insert a catheter and remove the needle ****************32-7************** |
|
|
caudal anesthesia
|
-produced by placing local in the caudal epidural space rather than the lumbar epidural space
|
|
|
confirming absence of intrathecal ot intravascular catheter placement
|
-aspiration to rule out intravascular injection (is not always reliable for CSF)
-give a test dose of local to rule out intrathecal injection -give a test dose of epinephrine to rule out IV injection |
|
|
dose of local for the test dose
|
bupivacaine 7.5 mg
OR lidocaine 45 mg |
|
|
epinephrine dose for test dose
|
15 ug
|
|
|
response to epi if it is IV
|
will get a sudden but transient increase in HR and BP, within 30-45 seconds
|
|
|
controversy in the use of epi for a test dose
|
-can have false-positives as the same changes can naturally occur in the woman due to contractions and whatnot
-might result in decreased uteroplacental perfusion -negative aspiration through a multiorifice catheter is considered by some to be adequate evidence you're not in a vein |
|
|
what should be monitored for after administering regional anesthesia?
|
-the regional can cause fetal bradycardia from uterine hyperactivity leading to decreased uteroplacental perfusion likely due to decreased circulating catecholamines from the block
|
|
|
treatment of this
|
nitroglycerine can achieve uterine relaxation if the hyperactivity persists
|
|
|
timing of epidural anesthesia during pregnancy
|
-can be done early in labor, and should not be withheld simply because an arbitrary amount of cervical dilation has not been achieved
|
|
|
does regional in early labor lead to a higher percentage of C-sections, and does it prolong labor?
|
-it does NOT lead to an increased rate of C-section
-it does NOT prolong labor, but actually may lead to a shorter duration of labor than systemic analgesia |
|
|
epidural during the stages of labor
|
-in early labor strive to give a segmental band from T10-L1 to cover the pain of uterine contractions and cervical dilation
-later in labor need to give a larger volume of local to provide perineal analgesia for SVD and if needed vaginal instrumentation -the catheter can be left in throughtout the active phase of labor and delivery, and postop for C-sections |
|
|
method of delivering local during epidural anestheisa for labor
|
-typically, after an initial bolus dose, local is infused continuously to give pain relief with similar or lower blood levels of local than would be seen with repetitive intermittent boluses
-the most important thing with this method is that the risk of total spinal if the catheter migrates intrathecally, or of cardiovascular collapse if IV is virtually eliminated: if it enters a vein there will be cessation of analagesia with no neuro or cardio effects, and if it is in the CSF will slowly get increasing motor and sensory blockade without risk of sudden total spinal |
|
|
PCEA
|
-alternative to continuos infusion or intermittent boluses, can program a baseline rate and additional patient administered optional amount at intermittent doses
-increases patient satisfaction and decreases workload on anesthesiologist |
|
|
choice of drug and dose in an epidural
|
-usually use dilute solutions of lidocaine, bupivacaine, ropivacaine, or chloroprocaine
-concentrations and volume of the loading dose given before the continuous infusion, and the rate of continous infusion vary |
|
|
drug used to relieve the visceral pain from the 1st stage of labor
|
-can usually be relieved by 6-10 mL of 0.125% to 0.25% bupivacaine or ropivacaine
-these drugs are chosen because of their long duration and relative motor sparing effect |
|
|
what do higher concwentrations of local lead to?
|
a more dense motor block
|
|
|
higher volumes?
|
more dermatomal spread
|
|
|
use of opioids for epidural
|
-can give reduced concentrations of anesthetics (0.0625% to 0.01% bupivacaine, and coadminister an opioid like fentanyl with the bolus and infusion
|
|
|
dose of fentanyl to give with the bolus
|
50-100 mcg
|
|
|
dose with the infusion
|
2 ug/ml
|
|
|
advantages of opioid use in the epidurals
|
-reduce the amount of motor block, which MAy allow women to ambulate
-preserve the perception of pelvic pressure with fetal descent -giving it with local may be synergistic |
|
|
so drug and dose of initial block
|
bupivacaine 0.125%- 0.25% 10-15 ml
bupivacaine 0.125% 10-15ml + fentanyl 50-100 mcg fentanyl 50-100 mcg (or sufentanil 10-15 ug) in 10ml saline |
|
|
subsequent doses
|
-can repeat the initial dose above intermittently
-can doa continuous infusion 10-15 ml/hr -bupivacaine 0.0625%- 0.125% + fentanyl 1-2 ug/ml (or sufentanyl 0.1-0.3 ug/ml) -bupivacaine 0.1%-0.25% without opioid -can add epi 1:400000 (2.5 ug/ml) to any of the above |
|
|
PCEA dose
|
initial bolus:
-bupivacaine 0.125%-0.25% (10-15ml) -bupivacaine 0.125% (10-15 ml) + fentanyl 50-100 ug -basal infusion 8 ml/hr (bupivacaine 0.08% + fentanyl 1-2 ug/ml) -demand bolus: 8 ml (bupivacaine 0.08% + fentanyl 1-2 ug/ml) -lockout interval 8 minutes -if require perineal anesthesia, give 10-15ml local (1-2% lidocaine or 2-3% chloroprocaine) |
|
|
choices of drug for combined spinal epidural to go intrathecally
|
can give opioid, a small dose of local, or both
|
|
|
intrathecal opioid in CSE
|
-the advantage is analgesia without motor blockade or sympathectomy, allowing for safe ambulation (if the epidural has not yet been activated)
-get side effects of nasuea, vomiting, and pruritis, but they are usually not substantial for lipid soluble opioids such as fentanyl or sufentanil -analgesia is limitied to the 1st stage of labor and about 2 hours of time, and is not effective in the 2nd stage of labor |
|
|
local given intrathecally in CSE
|
-gives more rapid segmental analgesia than epidural alone, and then epidural use after allows for continuation of this
|
|
|
spinal for vaginal delivery
|
-a spinal given right before vaginal delivery gives rapid onset of analgesia and skeletal muscle relaxation, which is esp important for forceps or perineal repair
-use 25-27 gauge pencil point needle to minimize spinal headache -using hyperbaric lidocaine 20-30mg, tetracaine 2-4mg, or bupivacaine 5-6mg gives rapid and profound analgesia to the perineal and vagina (can also do ropivacaine 0.5-0.75%) -maintain patient in sitting position for 1-2 minutes to limit sensory block to perineal analgesia (saddle-block) |
|
|
problem with true saddle-block
|
-does not produce complete analgesia because afferent fibers from the uterus are not blocked
|
|
|
modified saddle-block
|
-achieved by giving intrathecal larger doses of local (lidocaine 30-50mg, tetracaine 6-8mg) and maintaining the sitting position for 30 sec
-alows sensory anesthesia to extend to T10 or several levels higher preventing pain from uterine contractions |
|
|
spinal in advanced stages of labor
|
-give 2.5-5.0 mg isobaric bupivacaine and 10-25 ug fentanyl, which gives 90-120 min of analgesia without skeletal muscle weakness
|
|
|
contraindications to regional anesthesia
|
-patient refusal
-infection at the needle insertion site -coagulopathy -hypovolemic shock |
|
|
is HIV contraindicated in pregnant patients?
|
-no, and in fact if an epidural is needed for a blood patch to treat a puncture headache it is also OK
|
|
|
what is the most common complication of regional anesthesia for parturition?
|
systemic hypotension secondary to sympathetic nervous system block
|
|
|
measures to prevent and treat hypotension from regional in OB
|
prevention:
-adequate hydration -avoid the supine position, and left uterine displacement to prevent compression of the aorta and vena cava treatment: -further hydration and LUD -vasopressors -if treated promptly, the hypotension should not result in any fetal depression or neonatal morbidity |
|
|
intravascular injection of local
|
-of course can result in maternal seizures and CV collapse
-treat with resuscitation and support of maternal circulation, which will allow for adequate fetal blood flow and oxygenation |
|
|
plan for delivering the fetus if you suspect IV injection of local
|
-you should not deliver the fetus unless the mother's circulation cannot be resuscitated, because the neonate has a very limited ability to excrete local anesthetics, and could have prolonged convulsions once delivered
|
|
|
measures to minimize the risk of IV injection
|
-aspiration
-test dose -give the doses of local in incremental fashion |
|
|
excessive level of neural blockade
|
high/total spinal
-can occur during spinal, epidural, or caudal block, where the level of the local gets so high it blocks the motor fibers to respiratory muscles |
|
|
treatment of high spinal
|
-intubation and ventilation
-support maternal circulation by avoiding aortocaval compression, and fluids and pressors if needed |
|
|
what if all of these are not immediately effective in supporting the maternal circulation?
|
epinephrine (0.1-0.5 mg IV)
|
|
|
what if there is maternal cardiac arrest and resuscitation is unsuccessful?
|
-have to consider urgent delivery of the fetus
-if delivery is accomplished within 5 minutes of the arrest, the chance of the infant surviving is maximized; in addition delivery leads to emptying of the uterus, which relieves the aortocaval compression and improves the chances of maternal resuscitation |
|
|
factors of the progress of labor
|
include:
-increasing cervical dilation -effacement -descent over time |
|
|
variables affecting the progress of labor
|
-the progress of labor is unpredicatble because many factors influence it, including:
-pain -parity -presentation of the fetus -drugs and techniques used for analgesia and anesthesia |
|
|
anesthesia/analgesia and prolonging the latent phase of labor
|
-excessive sedation or premature initiation of regional anesthesia has been proposed to prolong the latent phase of labor, but evidence shows that the NEED for early IV or regional analgesia might be a characteristic of women who are going to have complicated/prolonged labor, and further labor can slow without any analgesia
-also, catecholamines in response to pain can actually inhibit uterine contractions |
|
|
most likely causes of delayed labor in the active phase
|
-cephalopelvic disproportion
-fetal malposition -fetal malpresentation |
|
|
impact of regional anesthesia on the active phase of labor
|
-is predictable: a T10 level from a spinal or epidural has no effect on the progress of labor (as long as there is no fetal malpresentation or hypotension)
-regional may, however, prolong the 2nd stage of labor by taking away the urge to bear down, however even if this stage is taken away there is no evidence of any adverse effect on the fetus |
|
|
effect on epidural anesthesia on maternal core body temperature
|
-causes an increase in temp due to several factors, including: most importantly presence of shivering, the duration of labor, and ambient temperature
|
|
|
progression of temperature change with epidural
|
-during the first 5 hours of labor, there is no change in body temp
-if labor is prolonged, body temp will rise 0.1C every hour, and can reach 38C by 12 hours |
|
|
effect of this temperature rise in patients who have prolonged labor
|
-it has been suggested that it leads to an increase in workups for fetal sepsis, though some studies show no increase
|
|
|
changes in management due to the increased temp
|
there dont need to be any:
-it is not associated with inc WBC count -infectious processes -and treatment is not necessary |
|
|
other nerve blocks for OB
|
include:
-paracervical block -pudendal nerve block -perineal infiltration |
|
|
paracervical block
|
-used for pain during the 1st stage of labor
-infiltrate local immediately lateral and posterior to the uterocervical junction to block pain transmission at the paracervical ganglion -anesthesia is less profound than with spinal or epidural, and only lasts 45-60 minutes, but dont get the problems associated with epidural/spinal (hypotension, hypoventilation, motor block) |
|
|
problems with paracervical block
|
-can get convulsion from systemic absorption, so need to closely monitor the fetus and be careful to aspirate before injecting, and wait 5 min before doing the other side
-this block is rarely performed because of the high incidence of fetal bradycardia, which is associated with increased neonatal morbidity and mortality, which is likely due to decreased uterine blood flow from the vasoconstriction caused by local |
|
|
so because of this, in who should this be avoided?
|
in patients with evidence of uteroplacental insufficiency or nonreassuring fetal heart tones
|
|
|
local chosen for paracervical block
|
-chloroprocaine is usually chosen because of its rapid breakdown if it gets IV
-can also use lidocaine -bupivacaine is CONTRAINDICATED |
|
|
pudendal nerve block
|
-done transvaginally with injection near the sacrospinous ligament, just medial and posterior to the ischial spine
-is used for vaginal delivery and vaginal assisted delivery with forceps, although the rate of failure is high, and even when the block works it is usually inadequate for forceps and even cervical exam |
|
|
perineal infiltration
|
-commonly done for episiotomy repair
-just need to take care not to inject into the fetal scalp or IV |
|
|
inhalational anesthesia for vaginal delivery
|
-some places use 50% nitrous oxide in oxygen, which if given without opioids gives adequate analgesia, yet is not sufficient to cause unconsciousness or loss of airway reflexes
-also maternal CV and respiratory depression is minimal, uterine contractility is unaffected, and neonatal depression does not occur -overall though, 50% nitrous is a weak analgesic |
|
|
what all women should recieve in preparation for c-section
|
-an oral nonparticulate antacid, such as sodium citrate (bicitra)
-some routinely give an H2 blocker (ranitidine) to reduce gastric acid, or metoclopramide to accelerate gastric emptying |
|
|
anesthesia for a c-section in a pregnant woman without an epidural catheter
|
spinal is the most common choice
|
|
|
advantages and disadvantages of spinal
|
-the block is easier than epidural, faster in onset, and more reliable at providing surgical anesthesia
-spinal headache incidence has been lowered with noncutting pencil point needles -maternal hypotension is more likely and more profound with spinal than epidural because the onset of sympathectomy is more rapid ( so just be aggressive with hydration, avoidance of aortocaval compression, and use of ephedrine) |
|
|
can spinal anesthesia be safely used in patients with preeclampsia?
|
yes
|
|
|
recommended drugs and doses for spinal snesthesia for c-section
|
local
-bupivacaine 12-15mg (1.6-2ml 0.75% in 8.25% glucose)(most common) -lidocaine 60-75 mg (1.2-1.5 ml 5% lidocaine in 7.5% glucose dilutaed with equal volume CSF) -tetracaine 8-10mg hyperbaric tetracaine (0.8-1 ml 1% tetracaine with equal volumes 10% glucose in water) intrathecal opioids -fentanyl 10-25 ug (0.1-0.5 ml of 50 ug/ml solution) -morphine 0.1-0.25mg (0.2-0.5 ml of 5mg/10ml) -can do fentanyl + morphine at above doses epinephrine 0.1-0.2 to prolong or improve the quality of block |
|
|
epidural for c-section advantages and disadvanmtages
|
-ideal for those who cant tolerate the sudden drop in BP associated with spinal
-attempt to get a level from T4 to the sacrum, but this may not be enough to aleviate the visceral pain associated with peritoneal manipulation, so may need adjuvant drugs |
|
|
epidural for c-section vs. for labor
|
-the technique, test dosing, and potential complications are similar, but the volume and concentration of the drugs for c/s are greater than need for labor
|
|
|
local for epidural for c/s
|
local
-lidocaine 1.5% to 2% plus epi 1:200,000 -bupivacaine 0.5% -chloroprocaine 3% |
|
|
test dose for epidural for c/s
|
-lidocaine 45 mg (spinal shock within 3-5 min)
-epi 15ug (HR inc within 60 sec) |
|
|
after a negative test dose
|
-give up to 20ml of the aforementioned local, in 5ml increments every 30 seconds
-give additional local if necessary to get a level of T4 |
|
|
Ph adjustment options for epidural
|
can add 1ml (1 mEq) sodium bicarbonate (8.4%) to 10 ml lidocaine
|
|
|
epidural opioid options
|
-fentanyl 50-100 ug
OR -sufentanyl 10-20 ug -morphine 4-5 mg |
|
|
suggested technique for general anesthesia for c/s
|
-give nonparticulate antacid (sodium citrate) within 15min of induction
-maintain LUD -start infusing crystalloid through a large-bore catheter -preoxygenate for 3 full minutes -when surgeon is ready to begin, have assistant apply cricoid pressure (and maintain it until the ETT position is verified and cuff is up) -give IV anesthetic and SCh 1-1.5 mg/kg, wait 30-60 sec, then intubate -give 50% nitrous with 0.5 MAC of volatile -begin deliberate hyperventilation -after delivery, can deepen anesthesia by increasing the [nitrous] or giving opioids, barbiturates, or propofol -extubate once pt is fully awake |
|
|
induction drugs for c/s
|
can use:
-thiopental -ketamine -propofol -etomidate |
|
|
what is the most commonly used IV drug for c/s?
|
thiopental, because it causes unconsciousness within 30 seconds
|
|
|
thiopental for c/s
|
-4-6 mg/kg IV
-the dose has no impact on fetal well-being (though at higher doses thiopental can cause neonatal depression, and cardiorespiratory support will be needed until the neonate is ready to excrete the drug, which may take up to 48 hrs) |
|
|
ketamine for c/s
|
-produces rapid anesthesia, and causes an INCREASE in BP, HR, and CO by sympathetic stimulation
-low doses (0.25 mg/kg IV) have profound analgesic qualities (unlike thiopental) -at excessively large doses can cause increased uterine tone and decreased uterine blood flow -can prevent the undesirable psychomotor effects by giving benzos |
|
|
in what situation do many anesthesiologists consider ketamine to be the drug of choice for inducing pregnant women?
|
-in those with active hemorrhaging
-uncertain blood volume -risk of profound hypotension in response to thiopental/propofol |
|
|
etomidate for c/s
|
-has rapid onset due to high lipid solubility, and has a short duration of action due to redistribution
-even though it has minimal CV effects, it (unlike thiopental or ketamine) is painful on injection, induces extrapyramidal motor activity, and is thus rarely used |
|
|
what is the factor that might limit the use of propofol in emergent c-sections?
|
the need to have induction agent predrawn up in syringes for rapid induction (not in reality, just in the book)
|
|
|
propofol for c-section
|
-like thiopental, is highly lipid soluble so results in rapid onset of action and rapid and complete recovery with less residual effect than thiopental
-not been shown to be superior to thiopental in maternal and neonatal outcome -further, has been associated with maternal bradycardia when given with SCh |
|
|
common maintenance of anesthesia for c-sections
|
is usually 50% nitrous combined with a low concentration of volatile
|
|
|
why is volatile anesthetic important?
|
the incidence of maternal recall is unacceptably high without it
|
|
|
opioids in a c-section under general
|
-are given after the baby is out to avoid placental transfer
|
|
|
placental transfer of volatile anesthetics
|
-is rapid since they are nonionized, highly lipid-soluble, low molecular weight molecules
-fetal concentrations will depend on the concentration and duration of anesthetic given to the mother |
|
|
if excessive concentrations of volatile are given to the mother for prolonged periods
|
-the neonate will show the effects of the drugs including flaccidity, cardiorespiratory depression, and decreased tone
-it is important to keep in mind though that if the depression seen in a neonate is due to the anesthetic drugs the infant is simply lightly anesthetized with inhaled anesthetic, and should respond to assisted ventilation to blow off the gas, which should occur quickly, and if it does not then other causes of neonatal depression should be sought |
|
|
fetal distress and the use of general anesthesia for c-sections
|
-it can sometimes be confusing what the cause of fetal depression is after delivering a previously depressed fetus via c-section under general
-the fact is, a depressed fetus is likely to be associated with a depressed neonate |
|
|
-what is the most important interval in a healthy fetus?
|
-the interval from uterine incision to delivery is more important than the interval from induction to delivery, since the former is the time when uterine blood flow is decreased, and fetal asphyxia
-the point is a long amount of time from induction to delivery will result in a lightly anesthetized neonate, not an asphyxiated one |
|
|
what is the neuromuscular blocker of choice for OB?
|
SCh, due to its rapid onset and short duration of action
|
|
|
SCh and the fetus
|
-under normal circumstances it is hydrolyzed by pseudocholinesterase in the maternal blood, and will not interfere with fetal neuromuscular activity
-if there is decreased or abnormal pseudocholinesterase, there can be prolonged maternal or neonatal respiratory depression from the muscle paralysis |
|
|
nondepolarizing NMBDs in OB
|
-since they are highly ionized and poorly lipid soluble they dont cross the placenta in amounts significant enough to cause skeletal muscle weakness in the neonate
-if however they are given in large doses over long periods it can occur |
|
|
diagnosing neonatal depression due to muscle blockade
|
-can be made on the basis of maternal history like prolonged administration of NMBD or history of pseudocholinesterase
-the response of the mother to the NMBDs -PE of the newborn: a paralyzed neonate will have normal CV function and good color, but no spontaneousventilatory movements or reflexes |
|
|
treatment of a neonate you suspect is paralyzed
|
-respiratory support until they can excrete all of the drug (can be up to 48 hrs)
-you can attempt to antagonize the NDMB with cholinesterase inhibitors, but adequate respiratory support is the mainstay |
|
|
fetal positioning
what is considered normal and abnormal? |
-fetal position is described based on the position of the fetal occiput, chin, or sacrum to the mother's left or right
-90% of deliveries are cephalic presentation with either occiput transverse or occiput anterior, so everything else is considered an abnormal presentation |
|
|
position of the occiput during normal delivery
|
-normally undergoes internal rotation to the OA position
|
|
|
what are the implications if this does not occur?
|
-the persistent OP position can cause prolonged and painful labor with back pain, likely due to the pressure of the occiput on the mother's sacral nerves
|
|
|
maternal and neonatal complications of breech deliveries
|
maternal
-cervical lacerations -retained placenta -hemorrhage neonatal -intracranial hemorrhage -umbilical cord prolapse (this is why most are delivered via c/s) |
|
|
regional anesthesia for c-section for breech presentation
|
-if planned can be done by either general or regional, but need to keep in mind that regional can cause difficulty getting the infant out of the uterine incision due to uterine hypertonus
|
|
|
treatment if there is trouble getting the infant out
|
-if uterine hypertonus is the cause, can give nitroglycerine (50 - 150ug) or metered dose sublingual nitroglycerine (400 ug per spray) to relax the uterus
-may need to rapidly induce geenral and intubate to get volatile anesthetic to relax the uterus |
|
|
anesthesia for planned vaginal delivery of a breech baby
|
-the most common approach is continuous epidural, which provides maximal perineal relaxation for delivery of the fetal head
-can use a low concentration of local (0.125% bupivacaine) to preserve the ability to push, and move to higher concentrations (2% chloroprocaine, 2% lidocaine) in the late 2nd stage to provide perineal relaxation for head delivery -if relaxation of the perineum is not adequate, may need to rapidly induce general and use volatile agent for this |
|
|
anesthesia for multiple gestations
|
-should be done in a place where an emergency c-section and neonatal resuscitation can be performed
-continuous epidural is a good choice, since it is flexible, but need to be mindful of the frequency of prematurity and breech presentation and possible need for emergent c-section -more than 2 fetal multiple gestations always require c-section |
|
|
pregnancy-associated hypertension
|
a range of disorders, including:
-gestational proteinuric hypertension -preeclampsia -eclampsia -hypertension occurs in 5-15% of all pregnancies, and is a major cause of obstetric and perinatal morbidity and mortality |
|
|
pathophysiology of pregnancy associated hypertension
|
involves nearly every organ system, including:
-cardiovascular -hepatorenal -pulmonary -CNS -intravascular fluid volume -coagulation -uterus |
|
|
cardiovascular system manifestations of preeclampsia
|
-generalized vasoconstriction
-increased vascular responsiveness to sympathetic nervous system stimulation -decreased uteroplacental perfusion |
|
|
hepatorenal system manifestations of preeclampsia
|
-decreased hepatic blood flow
-decreased GFR -decreased renal blood flow -retention of sodium and water |
|
|
pulmonary system manifestations of preeclampsia
|
-interstitial fluid accumulation
-decreased arterial oxygenation -exaggerated upper airway and laryngeal edema |
|
|
CNS manifestations of preeclampsia
|
-hyperreflexia
-edema -seizures |
|
|
intravascular fluid volume manifestations of preeclampsia
|
hypovolemia
|
|
|
coagulation manifestations of preeclampsia
|
-decreased platelet count
-increased fibrin split products |
|
|
uterine manifestations of preeclampsia
|
-hyperactivity
-premature labor |
|
|
preeclampsia
|
-syndrome starting after the 20th week of gestation characterized by:
-systemic hypertension (>140/90 mmHg) -proteinuria (>0.5 g/day) -generalized edema -headache |
|
|
what is a severe form of preeclampsia?
|
HELLP syndrome
-hemolysis (H) -elevated liver enzymes (EL) -low platelet count (LP) |
|
|
when do the manifestations of preeclampsia usually abate?
|
48 hours after delivery
|
|
|
eclampsia
|
-when seizures are superimposed on preeclampsia
-a potentially life-threatening condition |
|
|
common causes of maternal mortality in those with preeclampsia
|
-CHF
-MI -coagulopathy -cerebral hemorrhage |
|
|
what is the definitive treatment of preeclampsia?
|
delivery of the fetus and placenta
|
|
|
interim treatment of preeclampsia before this
|
-may need Mg and antihypertensive drugs
-should NOt use a regional or general anesthetic to treat the blood pressure, though if one is induced may need to lower the dose of antihypertensives |
|
|
Mg treatment
|
-acts to decrease the irritability of the CNS, decreasing the chance of seizures
-also decreases hyperactivity at the neuromuscular junction, likely by decreasing both the presynaptic release of ACh and the postsynaptic sensitivity to it -it also relaxes uterine and vascular smooth muscle increasing uterine blood flow |
|
|
how are the therapeutic effects of Mg assessed clinically?
|
-by monitoring DTRs, with marked depression indicating impending Mg toxicity
-also can periodically check serum Mg levels |
|
|
normal range of Mg serum levels
|
4-6 mEg/L
|
|
|
manifestations of Mg toxicity
|
-skeletal muscle weakness
-hypoventilation -cardiac arrest |
|
|
in what group of patients should Mg be carefully used in and why?
|
-since Mg is excreted by the kidneys, it should be acrefully titrated in those with impaired renal function, which by definition includes many patients with preeclampsia
|
|
|
Mg and anesthesia
|
-since it inhibits the release of ACh from presynaptic nerve terminals, it enhances the effects of nondepolarizing NMBDs
-in addition, preeclampsia is associated with decreased levels of plasma cholinesterase activity, so SCh and mivacurium can have prolonged effects independent of the Mg -Mg can also enhance the effects of sedatives and opiates, so their doses should be decreased |
|
|
Mg and the neonate
|
-it rapidly crosses the placenta, so the neonate can have decreased skeletal muscle tone
|
|
|
antihypertensive therapy for preeclampsia
|
-used when diastolic BP >110, with a goal of a diastolic around 100 mmHg
-most commonly used are hydralazine and labetolol |
|
|
what is the advantage of using hydralazine?
|
-its a vasodilator, so it increases uteroplacental and renal blood flow
|
|
|
dosing hydralazine
|
the peak isnt for 30 minutes, so be careful about giving doses during this time period
|
|
|
what might the presence of tachycardia with the hypertension necessitate that you use?
|
an adrenergic blocker, such as labetolol
|
|
|
what should be closely monitored during antihypertensive therapy?
|
the fetal heart rate, to look for early warning signs that the uteroplacental circulation is jeopardized due to decreased perfusion pressure
|
|
|
anesthesia for vaginal dleivery in a preeclamptic patient
|
-continuous labor epidural is acceptable if there is good medical control
-advantages are it decreases the need for systemic opioids and their deleterious effects on the fetus; it also decreases the likelihood of maternal BP increases in response to uterine contractions |
|
|
things to do in a preeclamptic woman before placing an epidural
|
-prehydration is often done as guided by clinical factors (UOP, oxygenation, exam, etc)
-in severely preeclamptic patients, need to check coagulation labs since platelat defects can occur, though this does not necessarily need to be done in mild preeclamptics |
|
|
treatment of maternal hypotension in preeclamptics
|
should decrease the doses of both ephedrine (2.5 mg IV) and phenylephrine (25 ug) since there is a presumed hypersensitivity of the maternal vasculature to catecholamines
|
|
|
planned c-sections in preeclamptic patients
|
-can be done with either spinal or epidural, with prompt treatment of hypotension with the reduced doses of vasopressors
|
|
|
emergency c/s in these patients
|
-when general is necessary, need to keep in mind the increased incidence of difficult intubation in preeclamptic pregnant patients
-may need to use smaller ETTs from the upper airway edema -usually do rapid sequence with thipental or propofol immediately followed by SCh -the response to laryngoscopy is exaggerated, so a short to of direct laryngoscopy is ideal |
|
|
preventing the sympathetic response to laryngoscopy in these patients
|
just before laryngoscopy, can use:
-nitroglycerine 1-2 ug/kg IV) -fentanyl 1 ug/kg IV -lidocaine 1.5 mg/kg IV -esmolol 1 mg/kg IV -also can use a remifentanil infusion |
|
|
other generla anesthesia considerations in these patients
|
-volatile anesthetic can be used to control hypertension intraoperatively
-remeber the enhanced effects the Mg has on NMBDs, so follow closely with a nerve stimulator -Mg also decreases uterine tone, giving a higher risk for bleeding |
|
|
treatment of decreased uterine tone in preeclamptics
|
-pitocin and PG F2a are safe to use
-methergine should be used with caution since it can precipitate a hypertensive crisis |
|
|
what is one of the leading causes of maternal mortality?
|
hemorrhage
|
|
|
what are the major causes of bleeding during the 3rd trimester?
|
placenta previa and abruptio placenta
|
|
|
what can be responsible for uncontrolled hemorrhage during labor?
|
uterine rupture
|
|
|
postpartum hemorrhage
|
-occurs in 3-5% of pregnancies
-can be due to: uterine atony, retained placenta, placenta accreta, or lacerations of the cervix or vagina |
|
|
placenta previa
|
-abnormally low implantation of the placenta in the uterus
|
|
|
symptoms of placenta previa
|
-painless vaginal bleeding, usually around 32nd week, when the lower uterine segment begins to form
|
|
|
anesthesia for c-section with placenta previa
|
-regional is fine
-may want 2 large bore IVs for rapid fluids/blood -in an emergency with active hemorrhage, may need to induce general, ketamine can be useful |
|
|
what characteristics are neonates delivered from mothers in hemorrhagic shock likely to have?
|
acidosis and hypovolemia
|
|
|
abruptio placenta
|
-separation of a normally implanted placenta after 20 weeks
|
|
|
signs of abruptio placenta
|
-if it only involves the placental margin, the bleeding will appear as vaginal bleeding
-if its involvement is deeper, large volume of blood can be concealed within the uterus |
|
|
what happens in these situations, when there is chronic bleeding and clotting between the placenta and uterus?
|
can lead to DIC
|
|
|
definitive treatment of abruptio placenta
|
evacuation of the uterus
|
|
|
anesthesia for abruption
|
-continuous lumbar epidural can be used for labor and vaginal delivery, as long as there are no signs of hypovolemia, clotting abnormalities, or fetal distress
-severe hemorrhage necessitates general (as with previa), (and as with previa the neonate may be acidotic and hypovolemic) |
|
|
causes of uterine rupture
|
is associated with:
-previous C-section with healed scar -rapid spontaneous vaginal delivery -excessive oxytocin use -with that said, 80% are of unknown cause |
|
|
vaginal delivery after c-section
|
-associated with 0.4-1% incidence of uterine rupture
-when this is planned, make sure to have full surgical team in place |
|
|
retained placenta
|
-occurs in 1% of vaginal deliveries
-requires manual exploration of the uterus -can use epidural or spinal if it was given for the delivery, if not can use IV opiates or nitrous oxide |
|
|
what if uterine relaxation is needed for a uterine manual exploration?
|
-nitroglycerine 50-150 ug IV
-rarely, may need to induce general anesthesia and use volatile agent to relax the uterus if it remains firmly contracted around the placenta |
|
|
uterine atony
|
-can cause hemorrhage immediately after delivery, or several hours later
-retained placenta commonly accompanies it - |
|
|
treatment of uterine atony
|
-with synthetic oxytocin, which do not contain vasopressin
|
|
|
giving oxytocin
|
-dilute solution will not exert any CV effects, but rapid IV infusion may be associated with tachycardia, vasodilation, and hypotension
-these are avoided by mixing it in a bag (20units per litre) and slowly infusing |
|
|
other drugs that can be used other than oxytocin for uterine atony
|
-methergine 0.2 mg IM
-prostaglandin F2a 0.25 mg IM -misoprostol, a PG E1 analog |
|
|
types of placental implantation beyond the endometrium
|
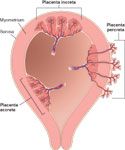
includes:
-placenta accreta: implantation onto the myometrium -placenta increta: implantation into the myometrium -placenta percreta: penetration through the full thickness of the myometrium |
|
|
placenta percreta complications
|
-it can implant into bowel, bladder, or other pelvic organs or vessels, any of which can produce a markedly adherent placenta which cannot be removed without tearing the myometrium
|
|
|
with what other abnormality do these abnormal implantation syndromes occur at a higher rate?
|
placenta previa
|
|
|
risks of placenta accreta
|
-occurs in 1 in 2500
-cannot be reliably diagnosed with US -the incidence is 5-7% in those with placenta previa and no prior c-section, but much higher if a previos c-section: with 1 previous uterine incision, 24-31%, with 2 ot more 50% |
|
|
anesthesia for accreta
|
-massive intraop blood loss is common, averaging 2000-5000 mL, with some pts needing more than 30 units of blood (coagulopathies develop in 20% of these)
-30-72% require cessarian hysterectomies -they say regional may be appropriate for most of these cases (but the likelihood of instability and need for massive transfusion may not make this realistic?) |
|
|
what would the sudden onset of repsiratory distress, systemic hypotension, and arterial hypoxemia signal?
|
amniotic fluid embolism
|
|
|
pathophysiology of amniotic fluid embolism
|
-unclear (since there are no animal models to show us)
-may be an anaphylactoid reaction to the amniotic fluid, causing pulmonary hypertension, right heart failure, subsequent biventricular cardiac failure likely from the ensuing arterial hypoxemia |
|
|
what patients are most likely to have amniotic fluid embolism?
|
-multiparous women with tumultuous labor
|
|
|
treatment of amniotic fluid embolism
|
-directed at cardiopulmonary resuscitation with inotropes and correcting any hypoxemia
-intubation and ventilation are almost always required |
|
|
what life-threatening event can happen with amniotic fluid embolism?
|
rapid onset of coagulopathy, and life-threatening hemorrhage
|
|
|
things that mimic amniotic fluid embolism
|
-venous air embolus
-PE -inhalation of gastric contents |
|
|
anesthesia for nonobstetric surgery during pregnancy
|
goals are to:
-avoid teratogenic drugs -avoid intrauterine fetal hypoxia and acdiosis -prevent spontaneous abortion early in pregnancy, and premature labor later on |
|
|
when is the critical period of organogenesis in humans?
|
between 15 and 56 days
|
|
|
anesthesia and teratogenicity
|
-there is no evidence that any of the currently used anesthetics are teratogenic
-evidence shows that exposure to nitrous does not alter outcome, and it is ok for in vitro fertilization and fallopian transfer procedures (though this is controversial) -no evidence drugs used for analgesia adversely affect the outcome of the baby |
|
|
how can intrauterine fetal hypoxia and acidosis be avoided?
|
-by avoiding maternal hypotension with LUD after 20th week
-also by preventing arterial hypoxemia and excessive changes in PaCO2 |
|
|
using high FIO2 in pregnancy
|
-does not increase the risk of in utero retinal fibroplasia (retinopathy) since the placenta consumed a lot of oxygen, and there is uneven distribution betwene the mother and fetal blood, and fetal PaO2 does not exceed 60 mmHg even when maternal PaO2 >500 mmHg
|
|
|
normal fetal umbilical vein blood gas readings
|
pH 7.35
PCO2 38 mmHg PO2 30 mmHg |
|
|
preterm labor in nonobstetric surgery
|
-the pathology requiring the surgery, not the anesthetic, has been associated with increased preterm labor, with intraabdominal procedures having a higher risk than peripheral
|
|
|
treatment of preterm labor after a surgery
|
-B2-agonists (terbutaline)
-Mg -indomethacin -Ca channel blockers (nifedipine) these all relax uterine smooth muscle, so lead to inhibition of contractions and increased uteroplacental blood flow |
|
|
side effects of B2-agonsits
|
-maternal hypokalemia and cardiac dysrhythmias
-fetal tachycardia and hypoglycemia |
|
|
anesthesia for nonobstetric surgery in a pregnant patient
|
-elective surgery should be deffered
-in need to operate, best to wait until 2nd or 3rd trimester -if need to in 1st, spinal seems advantageous because it limits fetal exposure to drugs -may want to consider continuous intraop monitoring after the 16th week -if use general, remember that volatile agents are NOT associated with a decrease in uterine blood flow if maternal systemic blood pressure is maintained normally -no matter what type of anesthetic is chosen, maintain [O2] at least 50% |
|
|
classification of fetal heart rate decelerations
|
as either late or variable
|
|
|
what relatively rare thing can be considered if fetal heart rate patterns are abnormal?
|
fetal scalp blood sampling for pH
|
|
|
fetal pH values
|
-the fetus should be considered depressed if pH around 7.0
-pH of 7.2-7.25 is associated witha vigorous infant at birth |
|
|
normal fetal pulse ox
|
>30%
|
|
|
normal fetal HR range
|
110-160 beat/min, with a beat-to-beat fluctuation of 5-20 beat/min
|
|
|
fetal heart rate variability
|
-reflects normal neural pathways from the cerebral cortex, through the medulla, vagus, and cardiac conduction system
-fetal well being is assured when there is good variability, and conversely absence of variability indicates fetal distress, as caused by hypoxia, acidosis, or CNS damage |
|
|
anesthesia and fetal HR variability
|
-opioids, anticholinergics, and general anesthesia seems to eliminate variability in the absence of fetal distress, which does not appear to cause long term problems except making it harder to interpret the fetal HR
|
|
|
late decelerations
|
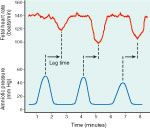
-slowing of the HR starting 10-30 sec after onset of contraction, likely mediated by chemoreceptors triggered by hypoxia
-characterized by a gradual onset of deceleration and return to basline, with the time from decel onset to nadir being >30 sec |
|
|
what are late decels associated with?
|
-fetal compromise (hypoxia and acidosis) likely due to myocardial hypoxia due to uteroplacental insufficiency, which can be caused by maternal hypotension, partial placental abruption, preeclampsia
|
|
|
interventions for late decels
|
-change in maternal position
-giving oxygen or tocolytics -promptly treat hypotension due to epidural with fluids and pressors |
|
|
variable decels
|
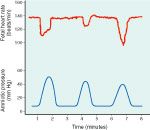
-vary in magnitue, duration, time of onset
-usually have a steep descent of fetal heart rate -thought to be caused by umbilical cord compresison |
|
|
meaning of variable decels
|
-unless last >30sec, or cause fetal bradycardia (<70) are usually benign
-changing maternal position generally stops them |

