![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
MOA of Latrunculin? |
It's binds to and sequester free Actin monomers so that they are out of the available pool and cannot be added to filaments. In other words, it effectively lowers the concentration of free monomers leading to actin depolarization. |
Uncle (latrUNCULin) thats ACTIN to get people (monomers) out of the POOL by grabbing (binding) to them. |
|
|
MOA of cytochalasin? |
Depolarizes actin filaments by binding to and capping the plus end of action filaments. Note: Will depolarize the minus end of a steady state until it reaches 0.6 (the minus end Cc). |
Stop CHALASIN (chasing) my end (+) with your CapZ on bro! |
|
|
MOA of phalloidin? |
Binds to sides of actin filaments and prevents depolymerization. |
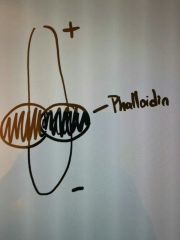
Like a the balls of a phallus, phalloidin binds to the sides...prevents shrinkage (aka. depolarization). |
|
|
MOA of thymosin? |
A protein that binds and sequester monomeric actin to prevent it from polymerizing (note: analogous to the drug latrunculin) |
Uncle (latrUNCULin) Thompson (Thymosin) is ACTIN to get people (monomers) out of the POOL by grabbing (binding) to them. |
|
|
MOA of profilin? |
Binds monomeric actin and induces the exchange of ADP for ATP. In this manner, it activates monomeric actin which stimulates filament growth. |

|
|
|
MOA of cofilin? |
Recycles actin monomers by binding to actin in the ADP state near the pointed/minus end and inducing depolarization. |
At the end (-) of his life, Akon (actin) is ripped into shreds and placed in a coffin (coffilin). |
|
|
MOA of CapZ? |
Binds to barbed/plus end of actin filament to block dynamics at that end (analogous to cytochalasin). Useful in inactive blood platelet cells. |
Stop CHALASIN (chasing) my end (+) with your CapZ on bro! |
|
|
MOA of gelsolin? |
Activated from Ca2+ release after the wounding of a blood vessel, gelsolin binds to the sides of the short actin filaments and severs them. This creates new plus ends not blocked by CapZ. |
A Cap wearing Zombie (CapZ), tried to make me bleed, but I kicked his hat off with my gel-soled shoes (gelsolin). |
|
|
MOA of filamin? |
Cross links actin filaments to form a 3D actin-gel that resists local mechanical pressures. |
Fill-em-in. Fills A In. "Fills actin in" by binding them together. |
|
|
MOA of fimbrin and villin? |
Connect parallel arrays of actin filaments to begin to form spike-like structures seen in an activated platelet. |
Fimbria and her Villains like ACTIN together to make the spikey things. |
|
|
MOA of ARP2/3? |
Serves as the major actin-nucleating factor (aka. the tiny seed required for the initiation of actin filament assembly) |
Assembling Red Pipes (ARP)! Actin is always red in the diagram. |
|
|
MOA of WASP/WAVE? |
Recruit ARP2/3 to nucleate actin filaments. |
WASPS Assemble Red Pipes (ARP). |
|
|
MOA of ActA? |
A transmembrane bacterial protein (eg. from Listeria) that is required for actin nucleation that results in propulsion of bacterial cell throughout the host cell. |
In Act A of the play, hysteria (Listeria) erupted when the character was shot out of a cannon! |
|
|
MOA of Dystrophin? |
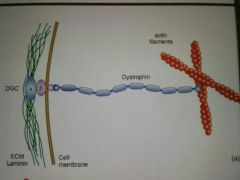
A spectrin-related protein that stretches from the interior actin filaments toward the plasma membrane, where it interacts with Dystrophin glycoprotein complex (DGC), which in turn spans the membrane and attaches to the ECM component laminin. |
The orphan felt he was in distopia,so he left Akon's house to become a Sheppard to watch over lambs. |
|
|
MOA of Nesprin-1? |
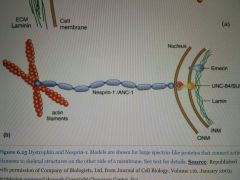
Connects the actin network in the cytoplasm to lamin forming the cytoskeleton inside the nucleus. |
Needing an Aspirin, Akon reached into Nicole's laminated cupboards. Aspirin = Nespirin-1 Akon = Actin Nicole = Nucleus Laminated = Laminin |
|
|
MOA of Griscelli syndrome (albinism)? |
Defect in motor myosin-Va which normally distributed pigment-containing melanosomes throughout the cell. As a result the melanosomes remain stuck near the Golgi, leading to albinism. |
The white witch Griselda confessed my 5 sins while eating a melon on a golf cart. Griselda = Griscelli syndrome White = Albinism My 5 sins = Myosin-5 Melon = Melanosomes Golf = Golgi |
|
|
MOA of Usher syndrome (deafness)? |
Mutations in myosin-VIIa disrupt the actin structures underlying the stereocilia in your ear, resulting in hearing loss. |
Usher confessed his 7 sins so loudly through the stereo that Akon lost his hearing. Usher = Usher's syndrome His 7 sins = Myosin-7 Stereo = Stereocilia Akon = Actin Lost his hearing = Deafness |
|
|
MOA of colchine (drug)? |
Binds to and sequester free a-/B-tubulin dimers, And prevents them from polymerizing. This effectively lowers the concentration of free dimers and leads to depolymerization. |
The coaching staff stole all the dimes sitting in the tub of water. |
|
|
MOA of taxol (drug)? |
Functions by binding to the side of a microtubule and stabilizes it to prevent depolymerization. |
It's very taxing to hold onto a heavy tube from the sides. |
|
|
y-tubulin ring complex (y-TuRC)? |
Consists of a number of proteins (the Dgrips) that form a ring that serve as the template off of which the 13 protofilaments of a microtubule can grow (note: located at the minus end). |
|
|
|
Catastrophe? |
The point at which a microtubule switches from growing to shrinking due to the plus end of the microtubule being in a GDP-bounds state. |
|
|
|
Rescue? |
The point at which a microtubule switches from shrinking to growing due to the plus end of the microtubule reestablishing it's GTP cap. |
|
|
|
MAP2/Tau? |
Stabilizes microtubules by binding to the side and increasing frequency of rescue events. |
According 2 the MAP they were lost. The only remaining chance of rescue was to attached a towel to the side of a long tube and wave it in the air! |
|
|
Kinesin-13? |
Induce catastrophes and shrinkage of microtubules. |
13...as in Friday the 13th is always a catastrophic day! |
|
|
Axonemal dyenin? |
Functions in eukaryotic cilia/flagella. Tail is attached to A tubule and it's three, ATPase-functioning heads move B tubule toward minus end on basal body. |
|
|
|
Dynein? |
Delivers cargo to minus end. |
|
|
|
Kinesin? |
Plus-end directed proteins (mostly). |
|
|
|
Vanadate? |
Inhibits dynein. |
You're so vain, that even when on a date all you could talk about was how you hated your dyed hair. |
|
|
Kinesin-5? |
Can push the two poles of a mitotic spindle apart when it cross-links antiparallel microtubules. |
Kyle's 5 sins were confessed between the two spinning flagpoles |
|
|
Kinesin-14? |
The exception to the rule. The only of its class that moves toward the minus end of microtubules. |
Put four teens together, and you'll get the exact opposite of what you want! |
|
|
UNC-83? |
A KASH protein responsible for nucleus migration in C. Elegans. It binds both kinesin-1 and dynein in order to switch between +/- end movements depending on the situation. |
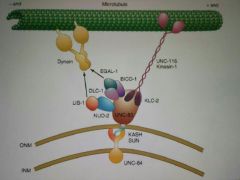
My cash-loaded uncle Nick just turned 83. All he can talk about are 1) his sins and about dying. |
|
|
Dam1? |
Forms a ring around a microtubule and is attached to the kinetochore by the Ndc80 complex. As the microtubules depolarizes, the ring is forced down the microtubule to the minus end. |
DaM1 that 80-year nudist, delinquent cop (NDC80), forcing his ring down my tubular throat! |
|
|
Plectin? |
A protein that joins intermediate filaments with either actin filaments or microtubules. |
Unlike electin intermediaries, plectin joins things together |
|
|
Chromokinesin? |
Generates the "polar ejection force" that move chromosomes toward the plus end of microtubules, away from the pole. |
Chromosome kinetics = movement of chromosome in opposite directions |
|
|
Restriction/START point? |
The "no turning back" point at which a cell decides to proceed to S-phase or to remain in G1-phase. |
|
|
|
CyclinD/CDK? |
Active at the end of G1 and regulates entry into the cell cycle. |
|
|
|
CyclinE/CDK? |
Regulates the initiation of S phase. |
Drunkard Elephants Appreciate Beer (CyclinD + E + A + B) |
|
|
CyclinA/CDK? |
Comes on in the early S phase and remains active until late G2 and prevents the reinitiation or S phase. |
|
|
|
CyclinB/CDK? |
Initiates mitosis. Also phosphorylates lamin, which leads to nuclear envelope breakdown. Also phosphorylates histone H1 and condensin, which initiates chromosome condensation. Also phosphorylates Separase to keep it inactive when Securin is absent. |
Such a Beezee! He broke Nicole's laminated envelope. Then condensed his 1 stone on someone's chrome bumper. He also securely separated his absent-minded friend. |
|
|
E2F? |
A transcription factor that controls the initiation of S phase by binding to the promoters of cyclinE and cyclinA, turning on their transcription, in late G1 phase. So, how is it regulated in early G1 phase? |
At the end of the G-string was Enough Cyclic Activity for Everyone2F! |
|
|
Rb? |
A protein that binds to and inactivates E2F during early G1 phase so cyclinE and cyclinA are not expressed. This protein itself is inactivated by phosphorylation from cyclinD/CDK. |
Ronaldo blocked (Rb) Everyone2Fu** |
|
|
Cdc6? |
A licensing factor for the firing of the origins of replication; the origin cannot fire without Cdc6 recruiting other components, but can also not fire until getting rid of Cdc6. As a result, at the beginning of S phase, it is quickly tagged by cyclinA/CDK and degraded. |
******* during cuddly sex (Cdc6) leads to a firing of the replication of the orgin of the human species. |
|
|
Cdc20? |
Once activated by CDK1, it's a subunit of APC that attaches multiple ubiquitins to lysine residues on cyclinB so it can be degraded. It also targets Securin. |
The center for disease control (Cdc20) is targeting 20 different circular Bacteria (cyclinB) by attaching ubiquitous (ubiquitin) antibiotics to induce lysis (lysine residues). |
|
|
Securin? |
A protein that binds to and inhibits Separase. |
|
|
|
Separase? |
A protease that cleaves Scc1. Is activated by kinetochore bipolar tension. |
To separate some clumsy chickens you do it bi pulling on their wings to create tension. |
|
|
Scc1? |
A protein that closes the cohesion ring that connects sister chromatids. |
|
|
|
Mad1? |
A protein that binds to unoccupied kinetochores, and then recruits and activates Mad2. |
For Kyle's to leave his number #1 chore unfinshed is pure MADness! |
|
|
Mad2? |
Once activated by Mad1, it functions to pause the cell cycle by inhibiting Cdc20. Once Cdc20 is stabilized, then Securin is stabilized and Separase remains inactive. |
The CDCs plan is MADness! It must be stopped! The bacteria must be Secured another way! |
|
|
ATR? |
A protein kinase that recognizes DNA damage and proceeds to phosphorylate a second kinase Chk1. |
Activate Total Repair! Check! |
|
|
Chk1? |
Delays the cell cycle by phosphorylating Cdc25... |
|
|
|
Cdc25? |
After being phosphorylated by Chk1, it then binds to protein called 14-3-3. 14-3-3 exports Cdc25 out of the nucleus and into the cytoplasm where it is inactive. Without Cdc25's activity, cyclinB/CDK remains inactive, giving time to the cell to repair it's DNA damage before entering mitosis. |
|
|
|
ATM? |
Recognizes DNA damage in G1 phase, then phosphorylates and activates Chk2, that phosphorylates and stabilizes p53. |
Activate Total Maintenance! Check#2! |
|
|
P53? |
It's a transcription factor, that when stable will activate p21, which is a CDK inhibitor. This slows down the cell cycle, giving time to repair the DNA damage. In other words, p53 is a tumor suppressor gene. Also, when a cell has too much damage, P53 will induce apoptosis. |
|
|
|
MDM2? |
MDM2 acts in opposition to P53 by inducing it's degradation. MDM2 acts as an oncogene. |
|

