![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Biotechnology (definition)
|
Any technological application that uses biological systems, living organisms or derivatives there of, to develop or make processes for specific use
|
|
|
Some uses of Biotechnology in Veterinary Medicine (6)
|
(1) PCR diagnostics for disease pathogens
(2) PCR diagnostics for breeding (3) Animal cloning/transgenics (4) Vaccine development (5) Therapeutic proteins (6) 'Gene therapy' |
|
|
Vectors (2 main types)
|
Vector (Definition): A DNA molecule used as a vehicle to artificially carry foreign genetic material into another cell, where it can be replicated and/or expressed. A vector containing foreign DNA is termed recombinant DNA. Vectors can be used to deliver therapeutic genes.
(1) Plasmid: Bacterial, extrachromosomal circular DNA, autonomously replicating. (2) Virus Vectors: Viruses genetically modified, used to deliver genetic material into cells. Killing ability of viruses can be useful for certain applications. |
|
|
Recombinant DNA
|
When two pieces of DNA of different origin are covalently linked together; the resultant molecule which does not occur naturally = recombinant DNA.
|
|
|
What's Required for Recombinant DNA (5)
|
(1) Pieces of DNA of interest (inserts)
(2) Vector; nucleic acid backbone, where want to insert specific gene, allows propagation of recombinant molecule (3) Restriction enzymes & ligase enzyme; way to join DNA (vector & insert) (4) Way to screen for the recombinant molecule (5) System to propagate/amplify recombinant molecule |
|
|
Restriction Enzymes
|
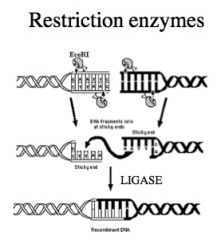
A class of enzymes that recognize short sequences of bases so as to make precise cuts at known points in both strands of a DNA molecule.
When vector DNA and donor DNA are cut with the same restriction enzymes, fragments contain matching "sticky ends" which can be joined together. |
|
|
Forming Recombinant DNA; inserting DNA fragment into plasmid.
|
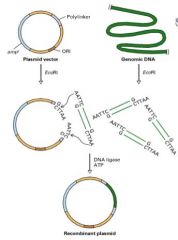
(1) Plasmid & DNA "digested" w/ same restriction enzymes
(2) Treated w/ enzyme T4 DNA ligase + ATP. This re-forms phosphodiester bonds and results in recombinant DNA plasmid. |
|
|
Ligase
|
An enzyme able to covalently seal cut pieces of DNA together (by synthesizing phosphodiester bonds b/w neighboring nucleotides), which requires energy from ATP.
Used to join vector DNA and donor DNA (when cut w/ same restriction enzyme) to form recombinant DNA. |
|
|
Isolating 'Insert' Sequences
|
(1) Protein expressing sequences (antigens, or therapeutic protiens), OR
(2) (Therapeutic) genes used for gene therapy... ...Can be isolated from (A) an organism's genome, or (B) from mRNA of organism Genome can be cloned into vectors, or mRNA converted to DNA and cloned into vectors. The clone is then selected which contains the target sequence. |
|
|
Libraries
|
A collection of DNA fragments that is stored and propagated through the process of molecular cloning.
There are different types of DNA libraries; including (1) genomic libraries (formed from genomic DNA), and (2) cDNA libraries (formed from reverse-transcribed RNA). |
|
|
Genomic Library
|
A genomic library is a collection of DNA (possibly divided into multiple fragments and packaged within cloning vectors such that each cloning vector carries a portion of the genome) that together represents the entire genome of a given organism.
Applications of genomic libraries include: (1) Determining the complete genome sequence of a given organism (2) Serving as a source of genomic sequence for generation of transgenic animals (3) Isolation and study of genes |
|
|
cDNA library
|
cDNA = "copy" of mRNA target(s). A sample of mRNA purified from a particular source (collection of cells, particular tissue, or entire organism), which has been converted back to a DNA template by enzyme REVERSE TRANSCRIPTASE. (It represents genes that were being actively transcribed in that particular source under the conditions that existed when the mRNA was purified.)
cDNA libraries only represent a very small portion of the overall genome. Applications of cDNA libraries include: (1) Sequencing of mRNAs (2) Isolation of protein coding sequence (3) Expression of protein |
|
|
Synthesis of cDNA (from mRNA libraries)
|
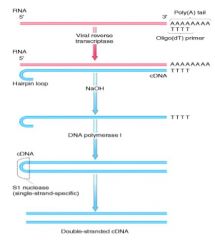
Method 1:
(1) DNA primer hybridizes to the polyA sequence (2) Enzyme REVERSE TRANSCRIPTASE (from retroviruses, which require for RNA to DNA conversion) is used to make the first strand of DNA (3) RNA is degraded by NaOH (4) Hairpin loop forms at 3' end, allowing DNA POLYMERASE I to synthesize the second strand (5) SINGLE-STRAND-SPECIFIC NUCLEASE treatment (S1 nuclease; nucleases cleave phosphodiester bonds b/w nucleotides) nicks the DNA resulting in production of double stranded cDNA. Method 2: By polymerase chain reaction (PCR) to make DNA copy of target sequence. Using primers, can amplify gene of interest. |
|
|
Polymerase Chain Reaction (PCR); Overview & Importance
|
A means by which low numbers of target DNA (or RNA) can be amplified to millions of copies of DNA.
(From a few copies to millions of copies of DNA, exponential amplification of DNA, to amounts detectable by agarose gel or other means.) Importance of PCR: (1) Basic research (2) Diagnostics (does animal have particular virus...) (3) Pharmaceutical/biotechnology industry (4) Forensic science (5) DNA sequencing |
|
|
Inputs for PCR (6)
|
(1) Target DNA or RNA in sample
(2) If target = RNA; involves REVERSE TRANSCRIPTASE PCR (RT-PCR) for RNA targets to convert to cDNA. (3) Primers; short sequences of DNA (oligonucleotides, about 20 nt) designed to match sequence on target DNA (to enable elongation/formation of complementary strand) (4) dNTP = nucleotides needed for copying DNA (5) Taq Polymerase = enzyme which amplifies DNA at high temperature (6) Thermocycler = instrument which cycles temperature facilitating different stages in reaction |
|
|
Steps to PCR (5)
|
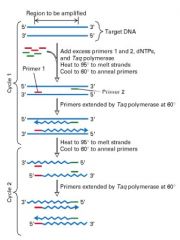
(1) Oligonucleotides are made that are homologous to the desired target sequence
(2) Target DNA is "melted" (95 degrees) to denature/split strands, then cooled (60 degrees) to anneal primers (3) Primers bind, then extend strand using Taq Polymerase (4) This cycle is repeated another 25 - 30 times (5) This produces a large amount of the desired target sequence (exponential increase) from finite amounts of starting DNA |
|
|
Electrophoresis (How to "see" DNA) (5 steps)
|
(1) Pour molten agarose gel (55 - 60 degrees) into a tray w/ comb. (Agarose acts as molecular "sieve" allowing separation of different sized DNA molecules)
(2) Remove comb from set gel, resulting in "wells" (3) Place DNA digested w/ restriction enzymes into wells of an agarose gel (4) Run electric current through gel to size separate DNA (5) Stain DNA with chemical ETHIDIUM BROMIDE, then visualize using UV LIGHT |
|
|
Recombinant Protein (definition)
|
A protein made from recombinant DNA (when pieces of DNA of different origin are covalently linked together).
|
|
|
Recombinant Protein Production (5)
|
(1) Start w/ pieces of DNA insert(s) (including gene encoding protein wanted to express) and vector (usually plasmid)
(2) Way to join them (restriction enzymes & ligase enzyme) (3) Way to screen for recombinant molecule (4) System in which to propagate recombinant protein (5) (Sometimes) way to recover and purify recombinant protein. |
|
|
WHEN Recombinant Proteins are Used (4)
|
(1) Basic research
(2) For preparation of antibodies (3) For direct treatments (Factor VIII for blood clotting) (4) For vaccination (both animals & humans) |
|
|
Interferon (IFN) ; definition & fxns (4)
|
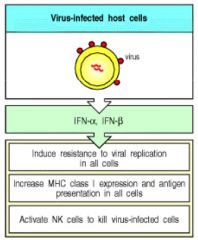
Proteins made and released by host cells in response to presence of pathogens (viruses, bacteria, parasites or tumor cells). They allow for communication b/w cells to trigger immune system.
IFNs belong to the large class of glycoproteins known as CYTOKINES. Functions of IFNs: (1) Interferons are named after their ability to "interfere" w/ viral replication (induce RESISTANCE to viral replication) w/in host cells, (2) Increase ability of uninfected host cells to resist new infection by virus (3) They activate immune cells (natural killer cells and macrophages), (4) Increase recognition of infection or tumor cells (by up-regulating antigen presentation to T-lymphocytes) production of IFNs during infection. |
|
|
Recombinant Interferons, definition
|
Recombinant interferon = interferon compounds (proteins) produced by recombinant techniques, where a gene of interest is placed into the genome of a system (cell culture or specific animal) & manipulation can lead to increased production of the gene product of interest. The final protein product = isolated and used for therapy.
The ability of interferons to help the body’s immune response means that recombinant interferon = used to treat many diseases that involve abnormal immune function. Using recombinant interferon = often referred to as immunotherapy. |
|
|
Recombinant Interferons, for DISEASE TMT
|
Recombinant interferons = used to treat diseases involving abnormal immune function, such as...
(1) Viral infections, including papillomaviruses, herpes virus, hepatitis B & C (2) Cancers, including leukemias and Kaposi's sarcoma in AIDS patients (3) Multiple sclerosis (using interferon beta) |
|
|
Chronic stomatitis; Cause & Tmt
|
Stomatitis = inflammation of mucous lining of mouth
Cause = Feline Calicivirus Tmt = Feline interferon omega (licensed as Virbagen omega) |
|
|
Recombinant proteins in VACCINATIONS
|
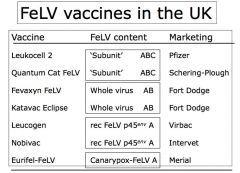
Recombinant proteins = made to be ANTIGENS of known virus, which stimulate immune response against antigen. Protects against disease caused by natural infection.
"Dead" or "non-living" vaccines. (Have some limitations compared to viral vector vaccines.) EXAMPLE; Feline Leukemia Virus (FeLV) subunit vaccines |
|
|
Pharming (definition)
|
The expression of recombinant proteins IN ANIMALS.
Most rProteins are produced bacteria/yeast. Advantages in animals = (1) SCALE OF PROD'N, (2) AUTHENTICITY of protein (post translational modification; PTM). Protein = produced in MILK of target animal, can be purified/isolated. |
|
|
PCR in Diagnostics; Advantages/Disadvantages & examples (3+)
|
Advantages; (1) Allows FAST diagnosis of disease (24 - 48 hours) (Much faster than cell culture virus isolation tests, 1 - 2 weeks), (2) By using strain specific primers can gain more information about pathogen causing outbreak.
Disadvantages; (1) Requires specialist lab, (2) Due to sensitivity, steps must be taken to avoid contamination of samples, (3) Existing primers may not recognize new strains (often primers designed to regions known to be conserved) Examples; (1) Canine Parvovirus, (2) Bovine Viral Diarrhea Virus (BVDV), (3) Feline; FHV, FeLV, FPV, FCoV (RT-PCR), Chlamidophila felis, Mycoplasma felis. |
|
|
Canine Parvovirus (CPV); PCR test
|
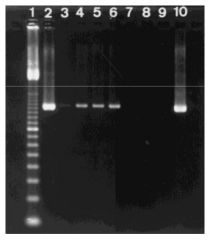
Pathogen of neonatal puppies, widespread domestic dogs, causes ENTERITIS (SI inflammation).
Single stranded DNA virus. Can be detected in fecal specimens using PCR. Confirming presence of CPV; Lane 1 = ladder / 2 lanes = reference strain / remaining lanes = clinical samples. Look at expected fragment size (1,266 bp). To confirm = use PROBES. |
|
|
Bovine Viral Diarrhea Virus (BVDV); PCR test
|
BVDV = from Pestivirus genus & Flaviviridae family. Infection in bulls = reduced semen quality, cows = poor conception rates & abortions.
Single stranded, positive sense RNA genome of 12.5 kb Can test for presence of virus in (1) semen, (2) serum, & (3) milk, using RT-PCR. |
|
|
Quantitative PCR tests
|
Indicate level of infection; how much pathogen was in initial samples.
|
|
|
Gene Therapy (what it involves & example diseases used for...)
|
(Similar principle as DNA vaccine...) Therapeutic gene = used for tmt of disease.
Involves; (1) Introduction of therapeutic gene to sufficient target cells, & (2) Expression of therapeutic gene at clinically effective level & for appropriate period. Gene therapy used for; (1) cancer, (2) arthritis, (3) cardiac disease, (4) single gene defects/replacement gene therapy |
|
|
Gene Therapy; 2 WAYS carried out...
|
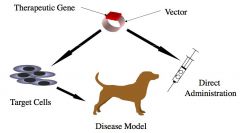
(1) IN VIVO; Directly apply to animal
(2) EX VIVO; Gene of therapeutic interest is introduced to isolated cells fm patient, genetically engineered cells are propagated & characterized, then injected/administered to patient. |
|
|
Ex-Vivo Gene Therapy
|
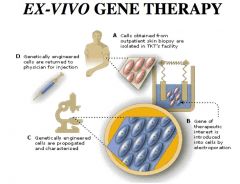
Cells isolated, therapeutic gene introduced. Genetically engineered cells = propagated & returned/injected back.
|
|
|
Pros & Cons of In Vivo Gene Therapy
|
PROS: (1) Directly apply to animal, (2) relatively inexpensive, (3) Potentially can attempt to target all cell types.
CONS: (1) Difficult to determine success rate, (2) difficult to target. |
|
|
Pros & Cons of Ex Vivo Gene Therapy
|
PROS: (1) Guaranteed good transgene expression, (2) Can target specific cell types, (3) Can multiply cells.
CONS: (1) Expensive, (2) Time consuming, (3) Not always appropriate to particular applications. |
|
|
IDEAL Gene Therapy Vectors
|
(1) Efficient
(2) Non-toxic (3) Targeted (4) Non-immunogenic (so immune system doesn't reject it) (5) high capacity (6) Easy to make (7) Stable Don't want to cause any harm to animal, and need long term expression, don't want immune system to remove vector. |
|
|
CHOICES for delivering gene therapy
|
Animal needs to be able to express the gene... there are several mechanisms to achieve that;
(1) VIRAL VECTORS; a) Retroviruses, b) Adenoviruses, c) Others (2) PHYSICAL METHODS: a) Ligand Conjugates, b) Liposome, c) Injection, d) Gene gun |
|
|
Pros & Cons of Viral Vectors (for Gene Therapy)
|
PROS; (1) Protect therapeutic gene well, (2) some specificity.
CONS: (1) Possible infection risk, (2) immune reaction (To virus coats, particularly adenoviruses. Which decreases ability to persist) |
|
|
Pros & Cons to Physical Methods (for Gene Therapy)
|
PROS: (1) Less hazardous (simpler) as there is no viral gene expression.
CONS: (1) Less efficient than viruses, (2) Poorer tissue specificity, (3) Safety issues; can it incorporate into DNA and cause cancer? |
|
|
Retroviruses as Viral Vectors for Gene Therapy
|
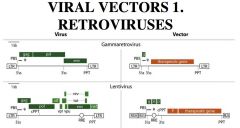
(1) Viral genes are replaced with therapeutic gene(s),
(2) GT (gene therapy) viral vectors are made in packaging cell line (or "helper" cell line"); in which missing viral functions are expressed by other recombinant vectors Gammaretrovirus; Most of the protein coding region is replaced with the therapeutic gene Lentivirus; Only affect non-dividing cells, which increases the advantage for gene therapy. |
|
|
Pros & Cons of RETROVIRUSES as Gene Therapy
|
PROS: (1) Replication of virus is defective, (2) Lentivirus (only); Infect a wide variety of dividing and non-dividing cells, (3) Integrate into host genome, (4) Long term transgene expression, (5) Low immunogenicity
CONS: (1) Retroviruses CAN cause insertional activation; (where insertion of virus into genome activates expression of an oncogene. Cancer outcomes depend on WHERE gene is inserted in host cell genome, if inserted in inappropriate part of genome) |
|
|
Adenoviruses as Viral Vectors for Gene Therapy
|

(1) First generation; involves E1 or E1 & E3 regions to be replaced with therapeutic genes
(2) 2nd generation; E2 and E4 deletions take place, resulting in complete replacement of viral genes. (3) Packaging lines are used to propagate viral vectors Same principle as retroviruses; knock out a few regions w/in viral genome. Yet more advanced/complex, need cell lines to be able to propagate. |
|
|
Pros & Cons of ADENOVIRUSES as Gene Therapy
|
PROS; (1) Very efficient at transducing (getting into) cells (for example, high viral titres), (2) Does not integrate into the genome, therefore no chance of insertional activation, (3) Large capacity for therapeutic gene insertion.
CONS: (1) Transgene expression is transient, (2) An immune response is mediated against the virus. |
|
|
Non-Viral Gene Therapy; PLASMID VECTORS
|
(1) LIPOSOME COMPLEXES; DNA is mied with lipids then applied,
(2) DIRECT INJECTION; naked plasmid DNA is injected directly into patient (3) GENE GUN; DNA is coated around very small metal beads and a "gun" is used to get particles into cells. (Driving high pressure gene therapy vectors into dermis of animal) |
|
|
Pros & Cons of PLASMIDS as Gene Therapy vectors
|
PROS: (1) Fewer safety issues than viruses, (2) Easier to prepare and more robust than viruses.
CONS: (1) Have to have direct access to target cells, (2) Transient expression, as does not integrate into the genome. |
|
|
What might we insert into a vector?
|
(1) Gene/cDNA encoding 'missing' protein; Replacement gene therapy (For examp, providing a correct copy of missing functional gene in genetic diseases associated w/ single gene)
(2) Gene/cDNA encoding protein; (a) proteins with modulating functions (often immune), (b) growth factors, (c) cell killing functions (with cancer applications, deliver genes to kill cancer), (d) antigens (vaccines) (3) Non-coding; Don't code for proteins; example Gene expression blockers (silencing RNAs that bind to genes to shut down transcription/translation for genes aberrantly expressed in certain individuals |
|
|
Most commonly applied vectors in GT trials
|
Top 3...
(1) Adenovirus, (2) Retrovirus, (3) Naked/Plasmid DNA (non-viral methods) |
|
|
Most common gene types transferred in Gene Therapy
|
Top 4...
(1) Antigen (2) Cytokine (small immune mediators) (3) Tumor supressor (4) Suicide genes (killing genes for promoting immune system response 2 cancer) |
|
|
Uses for Gene Therapy
|
(1) SINGLE GENE DEFECTS; such as cystic fibrosis, hemophilia, sickle cell anemia, immune deficiencies
(2) Diseases such as cancer & arthritis Top 3; (1) Cancer, (2) monogenic diseases, (3) Cardiovascular disease |
|
|
Why single gene defects are difficult to treat
|
(1) Requires expression of the corrected transgene in the appropriate cell type (for example, liver for Factor VIII)
(2) The transgene must be expressed indefinitely (3) Ideally, the trangene should be expressed at a physiological level (which might depend on what target cell type can get the vector into) (4) For a lot of single gene defects there are already effective therapies (Factor VIII for hemophilia.) Therefore, difficult to justify relatively "risky" gene therapy protocols. |
|
|
Why cancer is a "good" gene therapy target
|
(1) With terminal illness is easier to obtain regulatory approval for experimenting with gene therapy
(2) Gene therapy in cancer doesn't have to correct the genetic defect; can use a "killer gene" (to kill cancer cells if can target the vector correctly/specific to cancer cells) (3) Transient expression of the transgene is often sufficient to make an impact on disease |
|
|
Gene therapy setbacks;
|
(1) DEATH of a patient receiving gene therapy adenovirus; probably b/c of IMMUNE SYSTEM RESPONSE to virus (in 1999)
(2) LEUKEMIAS associated w/ retrovirus gene therapy; as direct result of where vector/virus was inserted (2000s) |
|
|
Gene Therapy Trial Progression
|
(4 Phases) 60% of clinical trials are in Phase 1, less than 20% progress to phase 3...
|
|
|
Veterinary Gene Therapy
|
In recent years, publications on gene therapy increased dramatically. Animals are good models to test gene therapies for humans on.
2 licensed gene therapies, vaccine form; (1) DNA Salmon vaccines (2) DNA pig GHRH Gene Therapy trials for cancers (equine and canine melanoma), as models for human disease |

