![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
What are enzymes? |
Enzymes are biological catalysts that speed up the rate of metabolic reactions by lowering the activation energy required to start the reaction. |
|
|
What are anabolic enzymes? |
Anabolic enzymes build larger products from smaller substrate molecules. |
|
|
What are catabolic enzymes? |
Catabolic enzymes break large substrate molecules into smaller products. |
|
|
What are extra-cellular enzymes? |
Some enzymes are secreted from cells by exocytosis and catalyse extracellular reactions.
|
|
|
Describe the structure of enzymes |
All enzymes are tertiary proteins where the polypeptide chain is folded back on itself into a spherical 3D globular shape held in place by tertiary bonding. The hydrophilic R groups are on the outside of the molecule, making it soluble.
|
|
|
Why do enzymes only catalyse one particular type of reaction?
|
Each enzyme is specific for one type of substrate. This means that each enzyme will only catalyse one particular type of reaction. This is because the enzyme has a specific shaped active site that has a complementary shape to only one type of substrate molecule.
|
|
|
How do enzymes break down molecules? |
Enzymes combine with substrate molecules at the active site, making temporary bonds to form an enzyme-substrate complex. The product is then released and the enzyme is left unchanged.
|
|
|
Describe the lock and key theory.
|
The enzyme has a specific shaped active site and a substrate with a complimentary shape enters.
Enzyme active site is a perfect shape match to the substrate molecule. Temporary bonds are formed. Product is released as it is a different shape to enzyme's active site. Enzyme remains unchanged. |
|
|
Describe the induced fit model.
|
When the substrate molecule combines with the enzyme it induces a small change of shape of the enzyme's active site; so that the active site changes to fit the substrate molecule perfectly. |
|
|
What does the induced fit model account for that the lock and key model doesn't?
|
The fact that a substrate can mould the enzyme to its own shape means that several different substrates can react with the same enzyme. This explains the broad specificity of some enzymes e.g. lipase.
|
|
|
What is lysozyme? |
Lysozyme is an anti-bacterial enzyme found in human tears, mucus and saliva.
|
|
|
What is lysozyme's function? |
To destroy pathogenic bacteria by breaking down their cell walls. |
|
|
Explain how lysozyme works. |
The active site is a groove, and sugars on the bacterial cell wall fit into it. The groove closes over the sugars, and the lysozyme molecule changes shape around the sugars and hydrolysis the bond holding them together. The cell wall is weakened; the bacteria absorb water by osmosis and burst.
|
|
|
Explain the role of activation energy in chemical reactions?
|
Molecules must have enough kinetic energy to approach each other closely enough to react. The minimum energy required for molecules to react, breaking existing bonds in the reactants and making new ones in the products, is the activation energy. |
|
|
Explain the relationship between activation energy and enzymes? |
Enzymes modify the substrate so the reaction requires a lower activation energy. When a substrate enters the active site of an enzyme, the shape of the molecule alters, allowing reactions to occur at lower temperatures, i.e. lower kinetic energy, than in the absence of enzymes. |
|
|
Described the course of an enzyme-controlled reaction? |
The progress of an enzyme-catalysed reaction for a given concentration of substrate can be followed by measuring either the formation of product or the disappearance of the substrate. The shape of the graph can be explained: - When the enzyme and substrate are first mixed together, there are many substrate molecules. - Both enzyme and substrate are in constant motion and collide. - Substrate molecules bind to the active sites of the enzyme molecules. In a 'successful' collision, substrate is broken down and products released. - More active sites become filled with substrate molecules. - The rate of reaction depends on the number of free active site, if all other conditions are optional and there is excess substrate. The enzyme concentration is the limiting factor because it controls the rate of reaction. - As the reaction proceeds there is less substrate and more product. The enzymes concentration is constant. The substrate concentration is the limiting factor because it controls the rate of reaction. - Eventually, all of the substrates has been used and no more product is formed so the line plateaus. The line goes through the origin because, at zero time, no reaction has happened yet. |
|
|
What are the factors that affect enzyme activity? |
Temperature pH Change in the three-dimensional structure of enzyme molecules. Substrate concentration Enzyme concentration |
|
|
What are intracellular enzymes, in solution? |
Intracellular enzymes act in solution inside cells. |
|
|
What are intracellular enzymes, membrane-bound? |
Intracellular enzymes may be attached to membranes inside the cell. |
|
|
Explain how temperature affects the rate of an enzyme controlled reaction. |
Increased temperature increases the kinetic energy of enzyme and substrate molecules and they collide with enough energy more often. This leads to more successful enzyme-substrate complexes being formed. Therefore, increasing the temperature increases the rate of an enzyme controlled reaction. This will continue until the optimum temperature is reached. This is where the maximum number of successful collisions and enzyme-substrate complexes form. Past the optimum temperature, the enzyme's active site becomes denatured as vibrations break hydrogen bonds within the active site of the enzyme, causing the shape of the active site to change. Less product is formed as successful enzyme-substrate complexes cannot form. This change in shape can be permanent. At low temperatures, the enzyme is inactivated as the molecules have very low kinetic energy. However, the shape is unchanged and the enzyme will work again if the temperature is raised. |
|
|
Describe what happens past an enzyme's optimum temperature. |
Past the optimum temperature, the enzyme's active site becomes denatured as vibrations break hydrogen bonds within the active site of the enzyme, causing the shape of the active site to change. Less product is formed as successful enzyme-substrate complexes cannot form. This change in shape can be permanent. |
|
|
Explain how pH affects the rate of an enzyme controlled reaction. |
Most enzyme's have an optimum pH, at which the rate of reaction is highest. Small pH changes around the optimum cause small reversible changes in enzyme structure and reduces its activity, but extremes of pH denature enzymes. |
|
|
Explain why pH affects the rate of an enzyme controlled reaction. |
The charges on the amino acid side-chains of the enzyme's active site are affected by hydrogen ions or hydroxide ions. At low pH, excess H+ ions are attracted to negative charges and neutralise them. At high pH, excess OH- ions neutralise the positive charges. This disrupts the ionic and hydrogen bonds maintaining the shape of the active site. The shape changes, denaturing the enzyme. No enzyme-substrate complexes form and enzyme activity is lost. |
|
|
Explain how substrate concentration affects the rate of an enzyme controlled reaction. |
If the enzyme concentration remains constant, the rate of reaction will increase as the substrate concentration increases. The reaction will level off once all the active sites are occupied; the number of available active sites become a limiting factor at higher substrate concentrations. When all the active sites are full the enzyme is said to be saturated. |
|
|
Explain why only a low enzyme concentration is needed to catalyse a large number of reactions. |
Once a product leaves the active site, the enzyme molecule can be re-used, so only a low enzyme concentration is needed to catalyse a large number of reactions. The number of substrate molecules that one enzyme molecule can turn into products in a given time is called the turn-over number. |
|
|
Explain how enzyme concentration affects the rate of an enzyme controlled reaction. |
As the enzyme-concentration increases, there are more active sites available and therefore the rate of reaction increases. |
|
|
What is an enzyme inhibitor? |
An enzyme inhibitor is a substance which combines with an enzyme and prevents it forming an enzyme-substrate complex. |
|
|
Explain how enzyme inhibition affects the rate of an enzyme controlled reaction. |
Enzyme inhibition is the decrease in rate of an enzyme-controlled action by another molecule, an inhibitor. An inhibition combines with an enzyme and prevents it forming an enzyme-substrate complex. |
|
|
What are the two types of inhibitors? |
Competitive and non-competitive. |
|
|
Explain competitive inhibitors |
Competitive inhibitors have a structurally similar shape to the substrate molecule; so they bind to the active site instead of the substrate molecule. A competitive inhibitor blocks the active site so prevents enzyme-substrate complexes forming. |
|
|
Explain non-competitive inhibitors |
Non-competitive inhibitors do not bind to the active site; they bind to a different part of the enzyme called the allosteric site. This binding alters the shape of the enzyme's active site so the substrate molecule can no longer fit into the active site. |
|
|
Explain how increasing the substrate concentration would affect the effectiveness of competitive inhibitors? |
Increasing the substrate concentration will decrease the effect of the competitive inhibitor as the enzyme is more likely to collide with a substrate molecule and form a successful enzyme-substrate complex. |
|
|
Explain how increasing the substrate concentration would affect the effectiveness of non-competitive inhibitors? |
Increasing the substrate concentration will not increase the rate of reaction in this case as the substrate can no longer fit into the enzyme's active site, Successful enzyme-substrate complexes cannot form. |
|
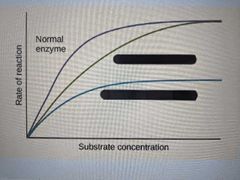
Which is a competitive inhibitor, which is a non-competitive inhibitor? |
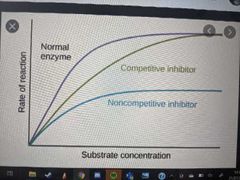
|
|
|
How are enzymes immobilised? |
Enzymes are immobilised when they are fixed, bound or trapped on an inert matrix. |
|
|
What are examples of how enzymes are immobilsed? |
Alginate beads. Gel membranes. |
|
|
Explain how enzymes are immobilised using alginate beads |
Enzymes are immobilised when they are fixed, bound or trapped on an inert matrix (alginate beads). These can be packed into glass columns. Substrate is added to the top of the column and as it flows down, its molecules bind the enzyme's active sites, both on the bead surface and inside the beads as the substrate molecules diffuse in. Once set up, the column can be used repeatedly. The enzyme is fixed and does not contaminate the products. The products are therefore easy to purify. |
|
|
Explain how enzymes are immobilised on a gel membrane (how it is more advantageous than using alginate beads) |
The enzyme can make direct contact with the substrate allowing the reaction to take place more quickly. (Substrate molecules myst diffuse into the jelly matrix of alginate beads, and the product needs time to diffuse out, so the reaction takes longer. Therefore, Enzymes immobilised in beads have a lower rate of reaction than those immobilised on a membrane. |
|
|
What are the advantages of using immobilised enzymes? |
- The enzyme does not contaminate the product. - The immobilised enzymes can be easily recovered and reused. - Only a small quantity of enzyme is needed. - The enzymes have greater stability and denature at higher temperatures. - Immobilised enzymes can catalyse reactions over a wider range of pH. - More than one enzyme can be used at the same time; enzymes can be added and removed - Greater control over the process - They can be used in a continuous process |
|
|
Explain why immobilised enzymes can catalyse reactions over a wider range of pH, at higher temperatures and in organic solvents. |
Enzyme instability is one factor preventing the wider use of enzymes that are free in solution. Organic solvents, high temperature and extremes of pH can all denature enzymes, with consequental loss of activity. Immobilising enzymes with a polymer matrix makes them more stable because it creates an microenvironment allowing reactions to occur at higher temperatures or extreme pHs than normal. Trapping an enzyme molecule prevents the shape change that would denature its active site, so the enzyme can be used in a wide range of physical conditions than if it were free in solution. |
|
|
In what industries are enzymes used? |
Food Pharmaceutical Agrochemical |
|
|
What are the uses of immobilised enzymes? (3 uses) |
Lactose-free milk Biosensors High-fructose corn syrup (HFCS) manufacture |
|
|
Explain how immobilised enzymes are used in the production of lactose-free milk. |
The milk is passed down a column containing immobilised lactase. The lactose binds tp the active sites on the lactase and is hydrolysed into its components, glucose and galactose. |
|
|
What are biosensors? (What do they do?) |
Biosensors turn a chemical signal into an electrical signal. Biosensors rapidly and accurately detect, identify and ,measure even very low concentrations of important molecules. |
|
|
Explain how immobilised enzymes are used in biosensors. Use the example of the detection of blood glucose. |
Biosensors work on the principles that enzymes are specific and are able to select one type of molecule from a mixture, even at very low concentrations. For example, when biosensors are used in the detection of blood glucose, the enzyme glucose oxidase, immobilised on a selectively permeable membrane placed in a blood sample, bind glucose. This produces a small electric current, detected by the electrode and read on a screen. Enzymes can also be immobilised onto test strips, where different strips may detect a variety of molecules. Testing strips with glucose oxidase immobilised onto them are used for detecting glucose in urine. |
|
|
Explain how immobilised enzymes are used in High-fructose corn syrup (HFCS) manufacturing. |

HFCS is manufactured in a multi-step process from starch. It uses several immobilised enzymes requiring different physical conditions. They include: |
|

Which line enzymes immobilised inside beads and which line is enzymes bound to gel membrane surface? |
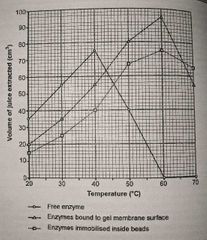
|
|

What is the risk assessment of this test. Give the hazard, risk and control measure of 2. |

|
|

What is the risk assessment of this test.Give the hazard, risk and control measure of 2. |

|

