![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
88 Cards in this Set
- Front
- Back
|
Prokaryotes |
Single cells organisms with no membrane bound nucleus or organelles |
|
|
Eukaryotes |
Multicellular organisms with membrane bound organelles |
|
|
Nucleus |
Contains DNA, directs the synthesis of all proteins required by the cell and therefore controls the metabolic activities of the cell |
|
|
Nucleolus |
Area within the nucleus responsible for producing ribosomes necessary for protein synthesis |
|
|
Mitochondria |
Site of the final stages of cellular respiration. Double membrane bound to produce cristae and the fluid interior called the matrix. Inner membrane contains enzymes for aerobic respiration. Also contains mitochondrial DNA to produce it's own enzymes |
|
|
Vesicle |
Membranous sacs for storage and transport |
|
|
Lysosomes |
Specialised vesicles containing hydrolytic enzymes |
|
|
Cytoskeleton |
Network of fibres which gives a cell shape and structure. Organelles held in place by the cytoskeleton. Composed of centrioles, microfilaments, microtubules and intermediate fibres |
|
|
Microfilaments (cytoskeleton) |
Contractile fibres of actin, responsible for cell movement and contraction during cytokinesis |
|
|
Microtubules (cytoskeleton) |
Globular tubulin proteins polymerise to form tubes that act as scaffold. They determine shape and acts a tracks for movement of organelles. Makes up spindle fibres |
|
|
Intermediate fibres (cytoskeleton) |
Give mechanical strength to cells |
|
|
Centrioles |
Component of the cytoskeleton, two associated centrioles form the centrosome involved in the assembly and organisation of spindle fibres |
|
|
Flagella Vs Cillia |
9+2 arrangement in structure Flagella: Whip like, longer but fewer in number, generally used for movement. Cillia: Hair like, used in sensory etc. |
|
|
Endoplasmic reticulum |
Network of membranes enclosib flattened sacs called cisternae. Connected to the nucleus. |
|
|
Smooth ER |
Responsible for lipid and carbohydrate synthesis and storage |
|
|
Rough ER |
Ribosomes bound to surface responsible for synthesis and transport of proteins. (Secretory cells contains more ER as they produce more enzymes etc.) |
|
|
Ribosomes |
No membrane, constructed of RNA. The site of protein synthesis |
|
|
Golgi Apparatus |
Similar in structure to SER. Modified and packages proteins into vesicles to leave the cell or lysosomes to stay in the cell |
|
|
Stages of Protein Synthesis |
1. Transcription 2. Translation 3. Proteins move to cisternae of RER and are packaged into vesicles 4. Vesicles move along the cytoskeleton to the Golgi apparatus 5. Vesicles fuse with the cis face of the Golgi and the proteins enter 6. The proteins are structurally modified such as adding carb groups etc. 7. Proteins leave the Golgi in vesicles pinched off at the trans face 8. Vesicles move towards and fuse to the cell surface membrane, releasing their contents by exocytosis. Or vesicles form lysosomes to remain in cell |
|
|
Transcription |
The copying of DNA base sequence in the nucleus using RNA, forming mRNA which leaves through the nuclear pores |
|
|
Translation |
Sequence of amino acids chained together at ribosomes to match mRNA triplet code |
|
|
Cellulose Cell Wall |
Rigid structure made of carbohydrate cellulose. Freely permeable. Contents of the cell push against the wall making it rigid. The wall acts as protection for the contented from invading pathogens |
|
|
Vacuoles |
Membrane lined sac in the cytoplasm containing cell sap. Most cells have large vacuoles to maintain rigor. Membrane is called tonoplast, it is selectively permeable. |
|
|
Chloroplasts |
Organelles responsible for photosynthesis. Double membrane bound structure similar to mitochondria. The fluid enclosed is called the stroma and they have an internal network of membranes called granum. Grana are joined by lanellae and the grana contain photosynthetic pigments |
|

Prokaryote/Eukaryote Comparison |

|
|
|
Elements in Carbohydrates |
Carbon, Hydrogen and Oxygen |
|
|
Elements in Lipids |
Carbon, Hydrogen and Oxygen |
|
|
Elements in Proteins |
Carbon, Hydrogen, Oxygen, Nitrogen and Sulfur |
|
|
Elements in Nucleic Acids |
Carbon, Hydrogen, Oxygen, Nitrogen and Phosphorus |
|
|
Uses of Water and the properties which lead to them |
• Solvent - many solutes in an organism can be dissolved in water • Coolant - High specific heat capacity • Transport Medium - Capillary attraction, adhesive and cohesive • Stable Environment for organisms - stable, doesn't change temperature or state easily • Insulation - Floating ice keeps lower water warm so aquatic organisms survive at lower temperatures |
|
|
α Glucose - Structure |
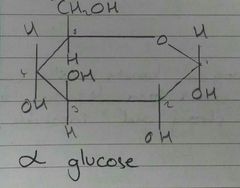
|
|
|
β Glucose - Structure |
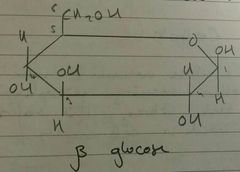
|
|
|
Glycosidic Bond |
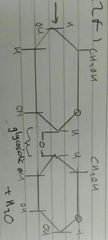
|
|
|
Cellulose Structure |
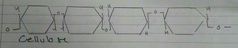
Alternating orientation of β Glucose. Cellulose form hydrogen bonds between each other forming microfibrils which form together to make fibres. This makes it strong and insoluble |
|
|
Monomers of Lactose |
Galactose and Glucose |
|
|
Starch |
Polymer of α Glucose Bonds between C1 - C4 Twisted with hydrogen bond supports |
|
|
Amylopectin |
Branched molecule Bonds between C1-C4 and C1-C6 |
|
|
Glycogen |
Polymer of glucose Branched, more than amylopectin so more compact Branching leaves many glucose open to be removed |
|
|
Hydrolysis |
Reaction which breaks down polysaccharides with the addition g water |
|
|
Test for Reducing Sugars |
Benedict's Test for Reducing Sugars: Place sample in boiling tube, grind and blend if necessary Add equal volume of Benedict's Reagent (alkaline solution of Copper II Sulfate) Heat mixture gently in boiling tube for 5mins
Results: Blue>Green>Yellow>Orange>Red No>High reducing sugars
Reducing sugars give up electrons to Cu2+ ions forming Cu+ hence colour change |
|
|
Test for Starch |
Iodine test for Starch: Mix a few drops of potassium iodide solution with food sample
Results: Positive when yellow/brown changes to purple/black |
|
|
Test for lipids |
Emulsion Test for Lipids: Mix food sample with ethanol Solution is then mixed with water and shaken
Results: White Emulsion - Lipids Present Remains Clear - No lipids |
|
|
Test for Protein |
Biuret Test for Proteins: Add liquid sample to test tube Add equal volume Biuret Reagent Shake mixture and leave to stand for 5mins
Results: Purple/Mauve - Proteins present No change - Proteins not present |
|
|
Saturated |
No double bonds present between carbon atoms |
|
|
Unsaturated |
Double binds present between some carbon atoms |
|
|
Triglyceride |
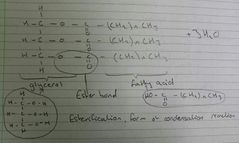
One glycerol molecule combined with three fatty acids |
|
|
Phospholipids |

Modified triglycerides which contain phosphorus. The phosphate ions are negatively charged and so soluble in water |
|
|
Sterols |
Steroid alcohols, type of lipid found in cells e.g cholesterol which regulates fluidity of phospholipid bilayer in membranes |
|
|
Roles of lipids |
• Membrane formation, creation of hydrophobic barriers • Hormone Production • Electrical insulation for nerve transmission • Waterproofing • Thermal insulation • Protection/cushioning of vital organs • Buoyancy |
|
|
Amino Acids Structure |
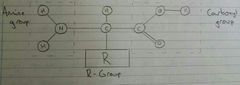
|
|
|
Peptide Bond |
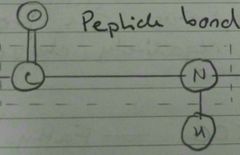
|
|
|
Primary Level of Protein Structure |
Sequence of amino acids, influence how peptide folds and it's final shape Only peptide bonds |
|
|
Secondary level of protein structure |
Oxygen, hydrogen and nitrogen interact Hydrogen bonds form H Bonds either pull together one chain into an α helix Or, multiple chains to form a β pleated sheet |
|
|
Tertiary level of protein structure |
Folding of protein into final shape Secondary structure brings R-groups close together to interact and fold Interactions between R-groups may be: - Hydrophobic/Hydrophilic interactions - Hydrogen Bonds - Ionic Bonds between +ve R-groups - Disulfide bonds, covalent bonds between R-groups with sulfur |
|
|
Quaternary level of protein structure |
Association of two or more individual proteins called sub units Interactions between sub units the same as tertiary but between peptide molecules |
|
|
Globular Proteins |
Compact and water soluble proteins Roughly spherical in shape Formed when tertiary structure keep Hydrophobic R-groups away from aqueous environment Hydrophilic R-groups on outer side of protein |
|
|
Conjugated Proteins |
Globular Proteins with a prosthetic group, non protein component |
|
|
Haemoglobin |
Quaternary conjugated protein made of 4 polypeptides Two α subunits, two β subunits. Each containing a haem group, the iron II ion can combine with oxygen |
|
|
Catalase |
Quaternary conjugated protein with four haem groups allowing it to catalyse breakdown of hydrogen peroxide. |
|
|
Fibrous Proteins |
Long insoluble molecules, due to high proportion of hydrophobic R-Groups. Tend to make long, strong molecules. |
|
|
Keratin |
Fibrous protein with large proportion of sulfur and disulfide bonds. Found in hair |
|
|
Elastin |
Fibrous protein, elastic fibres in alveoli and blood vessels |
|
|
Collagen |
Fibrous protein, rope-like connective tissue found in skin and tendons |
|
|
Nucleotide |
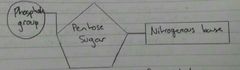
Monomers of nucleic acids Phosphodiester bonds form between nucleotides to produce nucleic acids |
|
|
DNA |
Deoxyribose Nucleic Acid Contains Penrose sugar - deoxyribose
Two types of bases: - Pyrimidines, smaller bases with single carbon ring. Thymine and Cytosine - Purines, larger bases with double carbon ring. Adenine and Guanine
Double helix structure, two strands run antiparallel (5' one end 3' at the other) to each other joined by hydrogen bonds
A - T (2 hydrogen bonds) C - G (3 hydrogen bonds)
|
|
|
RNA |
Ribose Nucleic Acid Ribose pentose sugar Thymine base replaced with Uracil U - A (2 hydrogen bonds) |
|
|
DNA Replication |
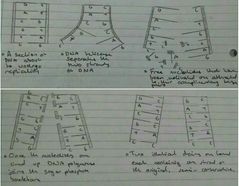
|
|
|
Codon |
A triple of bases which code for a single amino acid |
|
|
ATP |
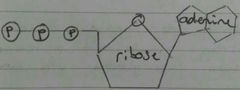
Adenosine Triphosphate Universal energy currency
Not good as a long term energy store as phosphate bonds are unstable
Attaching Pi to an ADP creates ATP. This is phosphorylation.
ATP + H2O -> ADP + Pi + Energy
Properties: - Small - Water soluble - Bonds between phosphates provide immediate energy - Release energy in manageable small quantities, energy is not wasted as heat - Easily regenerated
|
|
|
Enzymes |
Globular proteins which catalyse biological reactions Catalyse anabolic (building up) and catabolic (breaking down) reactions |
|
|
Lock and Key Hypothesis |
Active site on enzyme is complimentary to substrate, only specific substrates fit Substrate and enzyme bind to form enzyme-substrate complex Substrate(s) react and form enzyme-product complex The product leaves and the enzyme is not used so continues to function |
|
|
Induced Fit Hypothesis |
Active site changes shape slightly as substrate enters This puts strain on the substrates binds as a molecule This lowers activation energy for the reaction |
|
|
Intracellular Enzymes |
Enzymes which act within cells e.g. catalase |
|
|
Extracellular Enzymes |
Enzymes that act outside of cells e.g. amylase and trypsin |
|
|
Digestion of Starch |
Starch is broken down to maltose via amylase produced in the salivary glands and pancreas Maltose is broken down to glucose via maltase in the small intestine |
|
|
Digestion of proteins |
Trypsin, a protease, is produced in the pancreas and released in the pancreatic juice in the small intestine. It breaks down proteins into amino acids |
|
|
Competitive inhibition |
Molecule or part of a molecule with a similar shape to the substrate, fits in active site Reduces rate of reaction but does not reduce Vmax e.g. statins inhibit cholesterol synthesis so are prescribed to reduce cholesterol levels |
|
|
Non-competitive inhibition |
Molecule binds to other part of enzyme, an allosteric site This alters the tertiary structure and the active site is no longer complimentary Decreases rate of reaction and Vmax |
|
|
Cofactors |
A non-protein 'helper' component needed to carry out an enzymes function as a catalyst. If it is an organic molecule it is a coenzyme |
|
|
Precursor Activation |
A change in the enzymes shape required to be activated. Can be achieved by the addition of a cofactor or another enzyme cleaving of certain bonds to open up the active site |
|
|
Intrinsic Proteins |
Proteins imbedded in both layers of the phospholipid bilayer Channel proteins - allows passive movement Carrier proteins, active and passive transport |
|
|
Glycoproteins |
Used for cell signalling, receptors for neurotransmitters and peptide hormones |
|
|
Glycolipids |
Cell markers or antigens so it can be recognised by the immune system |
|
|
Facilitated diffusion |
Diffusion across a membrane through protein channels |
|
|
Active Transport |
Movement of ions from low concentration to high concentration, requiring energy and a carrier protein ATP bind to the carrier protein and is hydrolyse into ADP + Pi shape Molecule is released into the cell Binding phosphate causes the protein to change shapeMolecule is released into the cellPhosphate is released and joins to ADP to form ATPProtein returns to original shape Phosphate is released and joins to ADP to form ATP Protein returns to original shape |
|
|
Bulk Transport |
Movement of large molecules such as enzymes or whole cells such as bacteria Endocytosis (Phagocytosis - solids, pinocytosis - liquids), bulk transport into cells Exocytosis, bulk transport out |
|
|
Osmosis |
Diffusion of water across a partially permeable membrane from high water potential to low water potential |
|
|
Cell Cycle |
Interphase - Normal cell activity: Protein Synthesis Mitochondria grow and divide Chloroplasts grow and divide Normal metabolic processes G1 - First growth phase: Proteins from which organelles are synthesised are produced and organelles replicate Cell increases in size CHECKPOINT - Cell size, nutrients, growth factors, DNA damage S - Synthesis phase: DNA is replicated in the nucleus G2 - Second growth phase: Cell increases in size Replicated DNA is checked Energy stores increase CHECKPOINT - Cell size, DNA replication, DNA damage Final CHECKPOINT - Spindle assembly, check chromosomes are attached to spindle |

