![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
75 Cards in this Set
- Front
- Back
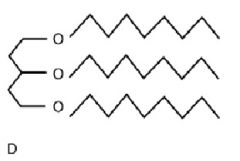
|
Lipid
|
|
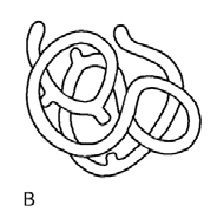
|
Functional Protein
|
|
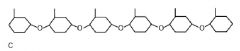
|
Nucleotide
|
|
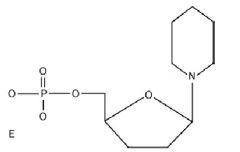
|
Polysaccharide
|
|
|
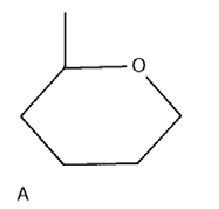
Monosaccharide
|
|
A:
|
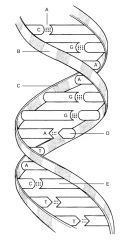
Hydrogen Bond
|
|
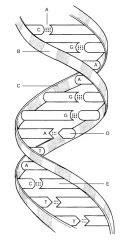
B:
|
Deoxyribose Sugar
|
|
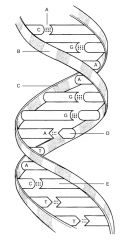
C:
|
Phosphate
|
|
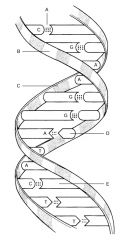
D:
|
Thymine
|
|
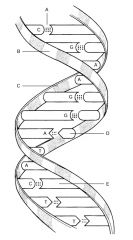
E:
|
Guanine
|
|
|
A bond in which electrons are shard unequally
|
Polar Covalent Bond
|
|
|
A bond in which electrons are lost or gained by atoms involved:
|
Ionic Bond
|
|
|
A bond in which electrons are equally shared:
|
Nonpolar Covalent Bond
|
|
|
A Type of bond important in tying different parts of the same molecule together into a three-dimensional structure:
|
Hydrogen Bond
|
|
|
Electrically charged particle due to a loss of an electron:
|
Cation
|
|
|
Neutral subatomic particle:
|
Neutron
|
|
|
Smallest particle of an element that retains its properties:
|
Atom
|
|
|
Smallest particle of a compound that still retains its properties:
|
Molecule
|
|
|
Water:
|
Compound
|
|
|
Carbon:
|
Element
|
|
|
Anything that occupies space and has mass:
|
Matter
|
|
|
Although a man who weights 175 pounds on Earth would be lighter on the moon and heavier on Jupiter, his _______ would not be different.
|
Mass
|
|
|
Is a function of, and varies with gravity:
|
Weight
|
|
|
A synthesis reaction always involves bond formation:
|
True
|
|
|
Chemical properties are determined primarily by neutrons:
|
False
|
|
|
A charged particle is correctly called an ion:
|
True
|
|
|
Isotopes differ from each other only in the number of electrons contained:
|
False
|
|
|
About 60-80% of the volume of livings cells consists of organic compounds:
|
False
|
|
|
Lipids are a poor source of energy:
|
False
|
|
|
Glucose is an example of a monosaccharide:
|
True
|
|
|
A molecule consisting of 1 carbon atom and 2 oxygen atoms is correctly written as CO2
|
True
|
|
|
The lower the pH, the higher hydrogen ion concentration:
|
True
|
|
|
Covalent bonds are generally weaker than ionic bonds:
|
False
|
|
|
Hydrogen bonds are strong bonds:
|
False
|
|
|
All organic compounds are covalently bonded:
|
True
|
|
|
In general, the category of lipids that we refer to as oils have:
|
High degree of unsaturated bonds
|
|
|
The genetic information is coded in DNA by:
|
The sequence of the nucleotides
|
|
|
Which of the following is not true of proteins:
|
They appear to be the molecular carriers of the coded hereditary information.
|
|
|
The single most abundant protein in the body is:
|
Collagen
|
|
|
Carbohydrates are stored in the liver and muscle in the form of:
|
Glycogen
|
|
|
The speed or rate of a chemical reaction is influenced by all of the following except:
|
The presence or absence of carbon
|
|
|
Salts are always:
|
Ionic Compounds
|
|
|
A solution that has a pH of 2 could best be described as being:
|
Acidic
|
|
|
Which of the following would be regarded as an organic molecule:
|
CH4
|
|
|
A chain of 25 amino acids would be called a:
|
Peptide
|
|
|
A long chain of simple sugars would be a:
|
Polysaccharides
|
|
|
Carbohydrates and proteins are built up from their basic building blocks by the:
|
Removal of water molecule between each 2 units.
|
|
|
Which statement about enzymes is false?
|
Enzymes raise the activation energy needed to start a reaction.
|
|
|
Select the statement that is most correct regarding chemical bonds.
|
Hydrogen bonds are very weak and often involve water.
|
|
|
Select the most correct statement regarding nucleic acids.
|
DNA is a long, double-stranded molecule made up of A, T, G, and C nucleotides.
|
|
|
Select the correct statement about isotopes.
|
Isotopes of the same element have the same atomic number but differ in their atomic weights.
|
|
|
The four elements that make up about 96% of body matter are:
|
carbon, oxygen, hydrogen, nitrogen.
|
|
|
Atom X has seventeen protons. How many electrons are in its valence shell?
|
7
|
|
|
If atom X has an atomic number of 74 it would have ________.
|
74 protons and roughly the same number of neutrons
|
|
|
The formula C6H12O6 means:
|
there are 12 hydrogen, 6 carbon and 6 oxygen atoms
|
|
|
The chemical symbol O = O means
|
the atoms are double bonded
|
|
|
Dipole is:
|
a polar molecule.
|
|
|
CH4 means
|
there is one carbon and four hydrogen atoms.
|
|
|
Amino acids joining together to make a peptide is a good example of a ________ reaction.
|
synthesis
|
|
|
The following is not an electrolyte
|
H2O
|
|
|
Grapefruit juice (pH 3) has ________ times the concentration of coffee (pH 5).
|
100
|
|
|
Sucrose is a ________.
|
disaccharide
|
|
|
Neutral fats have a ________ ratio of fatty acids to glycerol.
|
3:1
|
|
|
The atomic number is equal to the number of ________.
|
protons
|
|
|
Molecules such as methane that share electrons have ________ bonds.
|
covalent
|
|
|
Polysaccharides with long chains of similar units are called ________.
|
polymers
|
|
|
In a DNA molecule guanine would connect to ________.
|
cytosine
|
|
|
The ________ molecule directly provides energy for cellular work.
|
ATP
|
|
|
Starch is the stored carbohydrate in plants while ________ is the stored carbohydrate in animals.
|
glycogen
|
|
|
Explain the difference between potential and kinetic energy.
|
potential energy is the energy an object has relative to position. Kinetic energy is energy associated with moving.
|
|
|
What advantages does ATP have in being the energy currency molecule?
|
It is easy to store; it releases just the right amount of energy for the cell's needs so it is protected from excessive energy release. A universal energy currency is efficient because a single system can be used by all cells of the body.
|
|
|
Describe the factors that affect chemical reaction rates.
|
Temperature increases kinetic energy and therefore the force of molecular collisions. Particle size: smaller particles move faster at the same temperature and therefore collide more frequently; also, smaller particles have more surface area given the same concentration of reactants. Concentration: the higher the concentration, the greater the chance of particles colliding. Catalysts increase the rate of the reaction at a given temperature. Enzymes are biologically catalysts.
|
|
|
Protons and electrons exist in every atom nucleus except hydrogen. Is this statement true or false and why?
|
False - Hydrogen has one proton and one electron. It is the neutron that hydrogen does not have.
|
|
|
Name four things you know about enzymes.
|
1. They are proteins.
2. They have specific binding sites for specific substrates. 3. They lower the activation barrier for a specific reaction. 4. The names end in ase. 5. They can be denatured. 6. They can be used again and again. |
|
|
How can DNA be used to "fingerprint" a suspect in a crime?
|
The DNA of a person is unique to that individual. By obtaining the DNA from nucleated cells from the crime scene (e.g., tissue, sperm), heating it gently to separate the strands, combining it with the suspect's DNA, and allowing it to reanneal, the relatedness can be determined by the amount of base pairing.
|

