![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are amino acids? |
Amino acids are biologically important organic compounds composed of (-NH2 and (-COOH) functional groups, along with side chains specific to each amino acid. The key elements are carbon, hydrogen, oxygen and nitrogen |
|
|
|
Amino acids join via? |
Peptide bonds |
|
|
|
What are the classification of amino acids? |
Non-polar amino acidsPolar, uncharged amino acidsAcidic amino acidsBasic amino acids |
|
|
|
What are the non Polar amino acids |
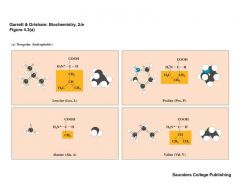
|
VALP |
|
|
What are the polar, uncharged amino acids |
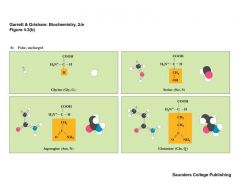
|
SAGG |
|
|
What are the Acidic amino acids |

|
TAGMIP |
|
|
What are the basic amino acids |
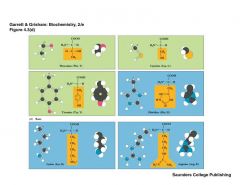
|
LACHTT |
|
|
List the uncommon amino acids |
- Hydroxylysine, hydroxyproline - collagen - Carboxyglutamate - blood clotting proteins - Pyroglutamate - bacteriorhodopsin - Phosphorylated amino acids - signaling device |
|
|
|
Describe the reactions of amino acids |

-Carboxyl groups form amides & esters -Amino groups form Schiff bases and amides *Side chains show unique reactivities -Cys residues can form disulfides and can be easily alkylated -Few reactions are specific to a single kind of side chain |
|
|
|
Describe the stereochemistry of amino acids |
-All but glycine are chiral -L-amino acids are predominate in nature -D, L- nomenclature is based on glyceraldehyde |
|
|
|
Describe the spectroscopic properties of amino acids |
- All amino acids absorb in infrared region - Only Phe Tyr and Try absorb UV - Absorbance at 280 nm is a good diagnostic device for amino acids - NMR spectra are characteristic of each residue in a protein, and high resolution NMR measurements can be used to elucidate 3-D structures of proteins |
|
|
|
Name two methods of separating amino acids |
- Ion exchange chromatography - High performance liquid chromatography |
|
|
|
List the essential proteins according to their properties |
branched chain: Val, Leu, Ilearomatic: Phe (→ Tyr), Trpbasic: His, Arg, Lys sulfur-containing: Met (→ Cys)other: Thr |
PVT TIM HALL |
|
|
List the non essential amino acids |
Gly, Ala, Pro, Ser, Tyr, Asn, Gln, Asp, Glu, Cys |
|
|
|
Proteins provide? |
- Amino acids for protein synthesis - Nitrogen atoms for nitrogen containing compounds - Energy when carbohydrate and lipid resources are not available |
|
|
|
Peptide bond? |
-Amide bond so stable - Amino group condenses with acid group - |
|
|
|
Describe the classification of amino acids |
- Simple: hydrolyze to amino acids only - Conjugated : bonded to non protein group such as sugar, nucleic acid, or lipid - Fibrous: long, stringy filaments, insoluble in water, function as structure - Globular: folded into spherical shape, function as enzymes, hormones, or transport proteins |
|
|
|
Describe primary structure |
Sequence of amino acids |
|
|
|
Describe secondary structure |
Interactions that occur between the c=o and N-H groups on amino acids Much of the protein core comprises helices and sheets, folded into a three-dimensional configuration: - regular patterns of H bonds are formed between neighboring amino acids- the amino acids have similar angles - the formation of these structures neutralizes the polar groups on each amino acid- the secondary structures are tightly packed in a hydrophobic environment- Each R side group has a limited volume to occupy and a limited number of interactions with other R side groups |
|
|
|
Describe Transamination |
Mechanism for conversion of non-essential amino acids into keto acidsEnzymes are aminotransferases or transaminasesTransfer of amino group from one amino acid into keto acid |
|
|
|
Describe tertiary structure |
Organization in three dimensions of all the atoms in the polypeptide A. Fibrous proteins B. Globular proteins |
|
|
|
Describe quaternary structure |
Confirmation assumed by a multimedia protein The individual polypeptide chains that make up a multimeric protein are often referred to as protein subunits. Subunits are joined by ionic, H and hydrophobic interactions |
|
|
|
Describe alpha helix |
Each carbonyl oxygen can form hydrogen bond with an N-H hydrogen on the next turn of the coil. |
|
|
|
Describe beta pleated sheet |
Each carbonyl oxygen hydrogen bonds with an N-H hydrogen on an adjacent peptide chain. |
|
|
|
List other secondary elements |
-random coil -loop -310 helix -B- hairpin -Paper clip |
|
|
|
Describe super-secondary structure |
There are some structural motifs in some proteins These structures can give important hints about protein function Eg, transmembrane domains Coiled coils Helix-turn-helix Signal peptides |
|
|
|
Describe two structural classification of proteins |
Type 1: Mainly alpha Mainly beta Alpha/beta Few secondary structure
Type 2: Class alpha Class beta Class a/B class A/b Membrane structure Multidomain |
|
|
|
Describe denaturation |
- Disruption of the normal structure of a protein, such that it loses biological activity. - Usually caused by heat or changes in pH - Usually irreversible |
|
|
|
List the steps for protein metabolism |
1) Transamination 2) Oxidative Deamination |
|
|
|
Describe Transamination |
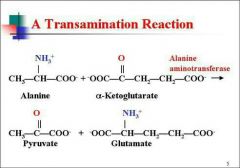
Mechanism for conversion of non-essential amino acids into keto acidsEnzymes are aminotransferases or transaminasesTransfer of amino group from one amino acid into keto acid |
|
|
|
Describe Oxidative deamination |

|
|
|
|
Summarize the urea cycle |

|
|

