![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
|
carboxyl
|

What is the name of this functional group
|
|
|
amido
|
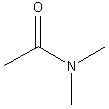
what is the name of this functional group
|
|
|
carboxyl
|
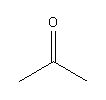
What is the class of functional group shown
|
|
|
A hydronium ion:
|
is a hydrated proton.
is a hydrated hydrogen ion has the structure H3O+. is the usual form of one of the dissociation products of water in solution. |
|
|
The pH of a sample of blood is 7.4. The pH of a sample of gastric juice is 1.4. The blood sample has:
|
a million times lower [H+] than the gastric juice.
|
|
|
The aqueous solution with the lowest pH is:
0.1 M HCl. 0.1 M acetic acid (pKa = 4.86). 0.1 M formic acid (pKa = 3.75). 10-13 M NaOH. |
0.1 M HCl.
|
|
|
The four covalent bonds in methane (CH4) are arranged around carbon to give what geometry?
|
tetrahedral
|
|
|
What is true of hydrogen bonds?
|
Hydrogen bonds form readily in aqueous solutions
Individual hydrogen bonds are much weaker than covalent bonds. Hydrogen bonds account for the anomalously high boiling point of water. In liquid water, the average water molecule forms hydrogen bonds with three to four other water molecules. |
|
|
Hydrophobic interactions:
|
are the driving force in the formation of micelles of amphipathic compounds in water.
|
|
|
Which of the following statements about buffers is true?
a. The pH of a buffered solution remains constant no matter how much acid or base is added to the solution. b. The strongest buffers are those composed of strong acids and strong bases. c. A buffer composed of a weak acid of pKa = 5 is stronger at pH 4 than at pH 6. d. When pH = pKa, the weak acid and salt concentrations in a buffer are equal. |
d. When pH = pKa, the weak acid and salt concentrations in a buffer are equal.
|
|
|
The Henderson-Hasselbalch equation:
|
a. relates the pH of a solution to the pKa and the concentrations of acid and salt.
|
|
|
What two properties of water are important for biological interactions?
|
the polarity of water and the cohesive properties of water
|
|
|
Consider an acetate buffer, initially at the same pH as its pKa (4.76). When sodium hydroxide (NaOH) is mixed with this buffer, the:
|
ratio of acetic acid to sodium acetate in the buffer falls.
|
|
|
At a pH of 12, what charged group(s) are present in glycine?
|
COO–
|
|
|
The angles of rotation about the peptide bond are called
|
phi and psi.
|
|
|
Name three amino acids that are positively charged at a neutral pH.
|
lys, arg, and his
|
|
|
The spatial arrangement of protein subunits is called
|
quaternary structure.
|
|
|
Why is the peptide bond planar?
|
It contains partial double bond character, preventing rotation.
|
|
|
The side chains of nonpolar amino acids are best categorized as:
|
uncharged.
|
|
|
In a highly basic solution, pH = 13, the dominant form of glycine is:
|
NH2–CH2–COO-.
|
|
|
The peptide alanylglutamylglycylalanylleucine has:
|
four peptide bonds.
|
|
|
Which of the following refers to particularly stable arrangements of amino acid residues in a protein that give rise to recurring patterns?
|
secondary structure
|
|
|
Which of the following describes the overall three-dimensional folding of a polypeptide?
|
tertiary structure
|
|
|
All of the following are considered "weak" interactions in proteins except:
a. van der Waals forces. b. hydrogen bonds. c. ionic bonds. d. peptide bonds. e. hydrophobic interactions. |
d. peptide bonds.
|
|
|
In the alpha helix the hydrogen bonds:
|
occur mainly between electronegative atoms of the backbone.
|
|
|
In an alpha helix, the R groups on the amino acid residues:
|
are found on the outside of the helix spiral.
|
|
|
Proteins often have regions that show specific, coherent patterns of folding or function. These regions are called:
|
domains.
|
|
|
oligomeric proteins:
|
Some subunits may have nonprotein prosthetic groups.
A subunit may be very similar to other proteins. Some oligomeric proteins can further associate into large fibers. Many have regulatory roles. |
|
|
how do enzymes stabilize a transition state?
|
covalent catalysis
using binding energy general acid-base catalysis catalysis by approximation |
|
|
A prosthetic group of a protein is a non-protein structure that is:
|
permanently associated with the protein.
|
|
|
Enzymes are potent catalysts. They:
|
lower the activation energy for the reactions they catalyze.
|
|
|
What is true of enzyme catalysts?
|
They lower the activation energy for conversion of substrate to product.
|
|
|
The role of an enzyme in an enzyme-catalyzed reaction is to:
|
increase the rate at which substrate is converted into product.
|
|
|
Which of the following statements is false?
a. A reaction may not occur at a detectable rate even though it has a favorable equilibrium b. At the end of an enzyme-catalyzed reaction, the functional enzyme becomes available to catalyze the reaction again. c. Substrate binds to an enzyme's active site. d. For S goes to P, a catalyst shifts the reaction equilibrium to the right. e. Lowering the temperature of a reaction will lower the reaction rate. |
d. For S goes to P, a catalyst shifts the reaction equilibrium to the right.
|
|
|
Which of the following parameters remains the same for S to P, whether the reaction is enzyme-catalyzed or uncatalyzed?
|
Keq'
|
|
|
Which of the following statements about a plot of V0 vs. [S] for an enzyme that follows Michaelis-Menten kinetics is false?
a. Km is the [S] at which V0 = 1/2 Vmax. b. The shape of the curve is a hyperbola. c. The y-axis is a rate term with units of mmol/min. d. As [S] increases, the initial velocity of reaction, V0, also increases. e. At very high [S], the velocity curve becomes a horizontal line that intersects the y-axis at Km. |
e. At very high [S], the velocity curve becomes a horizontal line that intersects the y-axis at Km.
|
|
|
The Lineweaver-Burk plot is used to:
|
solve, graphically, for the rate of an enzymatic reaction at infinite substrate concentration.
|
|
|
The double-reciprocal transformation of the Michaelis-Menten equation, also called the Lineweaver-Burk plot, is given by
1/V0 = Km /(Vmax[S]) + 1/Vmax. To determine Km from a double-reciprocal plot, you would: |
multiply the reciprocal of the x-axis intercept by -1.
|
|
|
The number of substrate molecules converted to product in a given unit of time by a single enzyme molecule at saturation is referred to as the:
|
turnover number.
|
|
|
In a plot of l/V against 1/[S] for an enzyme-catalyzed reaction, the presence of a competitive inhibitor will alter the:
|
intercept on the l/[S] axis
|
|
|
Which of these statements about enzyme-catalyzed reactions is false?
a. At saturating levels of substrate, the rate of an enzyme-catalyzed reaction is proportional to the enzyme concentration. b. The Michaelis-Menten constant Km equals the [S] at which V = 1/2 Vmax. c. If enough substrate is added, the normal Vmax of a reaction can be attained even in the presence of a competitive inhibitor. d. The rate of a reaction decreases steadily with time as substrate is depleted. e. The activation energy for the catalyzed reaction is the same as for the uncatalyzed reaction, but the equilibrium constant is more favorable in the enzyme-catalyzed reaction. |
e. The activation energy for the catalyzed reaction is the same as for the uncatalyzed reaction, but the equilibrium constant is more favorable in the enzyme-catalyzed reaction.
|
|
|
Vmax for an enzyme-catalyzed reaction:
|
is twice the rate observed when the concentration of substrate is equal to the Km.
|
|
|
Enzymes differ from other catalysts in that enzymes:
|
usually display specificity toward a single reactant.
|

