![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
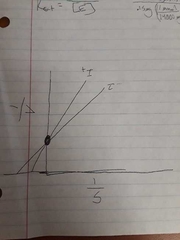
Competitive inhibition graph |
|
|
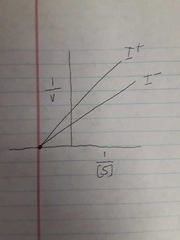
Graph of non competitive inhibition |
|
|
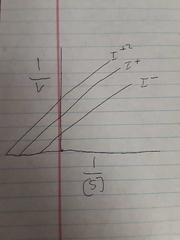
Graph of uncompetitive inhibition |
|
|
Competitive inhibition |
Only binds with free enzyme |
|
|
Noncomepetitive inhibition |
Inhibitor binds with any form of enzyme |
|
|
Uncompetitive inhibition |
Enzymes binds to enzyme substrate complex |
|
|
Oxidoreductase |
Redox reaction (reductase, hydrogenase, oxidase) |
|
|
Transferase |
Group transfer |
|
|
Hydrolase |
Bond cleavage with water |
|
|
Lyases |
Non oxidative, nonhydrolytic bond cleavage |
|
|
Isomerases |
Structure rearrangements |
|
|
Ligases |
Joining substrates using chemical energy |
|
|
Kcat |
= Vmax/[E] |
|
|
Promixity effect |
Substrates have to be close, in the right orientation and bump into each other to react. Enzymes help with this by creating a microenvironment for the substrates to increase their chances of interacting |
|
|
FDNB, dansyl chloride, and PITC |
All tell what the N terminus of the protein sequence |
|
|
Hydrazine and carboxypeptidase determine the |
C terminus of the protein sequence |
|
|
What amino acids does trypsin cleave after? |
Lys and arg |
|
|
What amino acids does chymotrypsin cleave after |
Aromatics, phe, tyr, trp |
|
|
What is the inverted Michaelis-Menten equation |
1/v= (Km+1)/(Vmax*[S]) + 1/Vmax |
|
|
In competitive inhibition is the Km value the same or different with the inhibitor? |
Km is different, typically smaller |

