![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
|
Catalyst
|
• a chemical agent that speeds up a chemical reaction without being consumed in the process
• ENZYMES!! • Without enzymes there would be a build up of reactants and not enough products |
|
|
The chemical reaction curve shows
|
1) Reactants = starting material
2) Products = end result 3) Transition state = old bonds break and new bonds form from heat creating entropy 4) Energy of activation (Ea) = heat energy absorbed by the surroundings that breaks old bonds b/c molecules become high in energy and unstable |
|
|
Which bonds need enzymes to break?
|
non-polar covalent bonds
• they are the strongest b/c they cannot break in an aqueous solution, while ionic and polar can |
|
|
How do enzymes speed up chemical reactions?
|
They lower the energy of activation by requiring less heat
|
|
|
Active site
|
the groove on the enzyme where the reaction occurs and is specific to a particular substrate
|
|
|
Substrate
|
the reactant which enters the active site forming a substrate-enzyme complex
|
|
|
Induced fit
|
when the substrate enters the enzyme's active site the enzyme undergoes a conformational change in shape by wrapping around the substrate
|
|
|
What causes enzymes to lower energy of activation?
|
When a substrate enters an active site and the enzyme undergoes induced fit, the enzyme is crushing the substrate to break its bonds
• crushing = the chemical groups of the substrate interact with the R-groups of the amino acids in the active site ➞ strains the bonds • therefore more bonds are broken quicker with less heat |
|
|
Do enzymes get used up breaking down substrates?
|
No! Soon as a reaction is over they can be used again
|
|
|
Sucrase
|
an enzyme that breaks down the carbohydrate/saccharide dimer sucrose (glucose + fructose)
• enzymes usually end in "ase" |
|
|
Factors that adversely affect enzyme activity
3 What can happen to the enzymes? |
1) Temperature too high
➞ lower temps make reactants move slower, but rxn still occurs ➞ higher temps denature enzymes 80-100 ºC 2) pH too high or low 3) High salt concentration They can denature |
|
|
What is the optimum temperature for enzymes in the human body?
|
37ºC ➞ 98.6ºF
• brings reaction to transition state • lower temps |
|
|
What is the optimal pH for enzymes?
|
usually 6-8
for pepsin pH 2 |
|
|
Cofactors vs. coenzymes
|
• Both are enzyme helpers
• they enter the active site along with the substrate and aid in the catalytic rxn Cofactors • inorganic substances • ex. Cu, Zn, Ca, Fe Coenzymes • organic substances • ex. most vitamins and modified nucleotides (NAD+) |
|
|
Competitive vs. non-competitive inhibitors
|
Competitive:
• has a similar shape to the substrate • enters active site so substrate cannot bind • reversible if you increase the substrate Non-competitive: • binds to another place on the enzyme • changes the shape of the active site so the substrate cannot fit |
|
|
Allosteric enzymes
2 types |
Molecules that bind to an enzyme, changing its shape
Activators: • bind to an inactive enzyme changing it to its active form so that substrates can bind Inhibitors: ª bind to an active enzyme changing it to its inactive form so that substrates cannot bind • non-competitive inhibitor |
|
|
Negative feedback
|
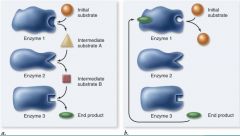
When there is enough product produced, so that the product becomes an allosteric inhibitor and ***** down the whole system
|

