![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
303 Cards in this Set
- Front
- Back
|
what enzyme is deficient in patients with lactose intolerance?
|
lactase
|
|
|
what is the inheritance of congenital lactase deficiency?
|
autosomal recessive
|
|
|
what are 4 causes of lactose intolerance?
|
(1) Lactase deficiency
(2) Celiac disease (3) Starvation (4) Gastroenteritis |
|
|
what enzyme is deficient in fructose intolerance and what reaction would it normally catalyze?
|
* Deficient enzyme: Aldolase B
* Aldolase B converts fructose-1-phosphate to DHAP and glyceraldehyde |
|
|
three symptoms of fructose intolerance?
|
(1) Hypoglycemia
(2) Jaundice (3) Vomiting |
|
|
what is the other name for fructose intolerance?
|
hereditary aldolase B deficiency
|
|
|
in patients with galactokinase deficiency, what accumulates in the eye lens and causes cataracts?
|
galactitol
|
|
|
what reaction is normally catalyzed by galactokinase?
|
conversion of galactose to galactose-1-phosphate
|
|
|
what enzyme is deficient in galactosemia and what reaction does the enzyme normally catalyze?
|
Patients with galactosemia are deficient in the enzyme uridylyltransferase
Uridylyltransferase normally catalyzes conversion of galactose-1-phosphate to glucose-1-phosphate |
|
|
what condition results from a reduced ability to convert galactose-1-phosphate to glucose-1-phosphate?
|
galactosemia
|
|
|
what enzyme converts galactose-1-phosphate to glucose-1-phosphate?
|
uridylyltransferase
|
|
|
what is the treatment for galactosemia?
|
exclude lactose from diet
|
|
|
what is the most common genetic deficiency in the world?
|
glucose-6-phosphate dehydrogenase deficiency
|
|
|
what compound biulds up in RBC's as a result of glucose-6-phosphate dehydrogenase deficiency?
|
H2O2
|
|
|
what reaction is normally catalyzed by glucose-6-phosphate dehydrogenase?
|
conversion of glucose-6-phosphate to 6-phosphogluconolactone together with reduction of NADP+ to NADPH
|
|
|
what are three anti-thiamin factors and how do they lead to thiamin deficiency?
|
(1) Ethanol: inhibits absorption of thiamin
(2) Raw fish: thiaminase (heat labile) breaks down thiamin (3) High stomach pH: thiamin is unstable at high pH |
|
|
what is primary thiamin deficiency?
|
low intake of thiamin
|
|
|
what condition is caused by thiamin deficiency?
|
Beriberi
|
|
|
what causes Beriberi?
|
thiamin deficiency
|
|
|
in one word each, differentiate between wet and dry beriberi
|
Wet Beriberi = cardiovascular
Dry Beriberi = CNS |
|
|
4 symptoms of wet beriberi
|
(1) Edema
(2) Enlarged heart (3) Tachycardia (4) Weakness |
|
|
what is the cofactor form of the vitamin that is deficient in patient with Beriberi?
|
Vitamin B1 = thiamin = thiamin pyrophosphate (TPP)
|
|
|
what condition results from Dry Beriberi?
|
Wernicke-Korsakoff syndrome
|
|
|
4 symptoms of Wernicke encephalopathy
|
(1) Ataxic gait
(2) Horizontal nystagmus (3) Uneven pupillary size (4) Confusion |
|
|
3 symptoms of Korsakoff syndrome
|
(1) Memory loss
(2) Confabulation (3) Hallucinations |
|
|
what condition results from vitamin B1 deficiency?
|
Beriberi
|
|
|
whenever thiamin deficiency is a possibility, what and how should treatment be administered?
|
thiamin should be given BEFORE carbohydrate loading
|
|
|
what is the treatment for genetic deficiency of pyruvate dehydrogenase?
|
ketogenic diet high in fat and the ketogenic amino acids leucine and lysine
|
|
|
what genetic deficiency can produce similar symptoms to dry beriberi?
|
genetic PDH deficiency
|
|
|
how many ATP are produced in the ETC from NADH and FADH2
|
NADH = 3 ATP
FADH2 = 2 ATP |
|
|
mechanism of Amytal
|
prevents electron shuttling from complex I to CoQ
|
|
|
what is amytal?
|
Barbituate that prevents shuttling of electrons from complex I to CoQ, that is used in treatment of insomnia, anxiety, and prolonged seizures
|
|
|
barbituate that prevents shuttling of electrons from complex I to CoQ
|
amytal (amobarbital)
|
|
|
lipophilic pesticide capable of crossing the BBB that blocks shuttling of electrons from complex I to CoQ
|
Rotenone
|
|
|
mechanism of Rotenone
|
blocks electron transport from complex I to CoQ
|
|
|
for what is Rotenone used?
|
pesticide
|
|
|
mechanism of cyanide
|
blocks cytochrome C oxidase
|
|
|
What drug is a potent vasodilator used to treat malignant hypertension that can cause CN poisoning in overdose?
|
sodium nitroprusside
|
|
|
what condition is treated with sodium nitroprusside?
|
malignant hypertension and acute angina
|
|
|
what drug is an uncoupling drug used by body builders to reduce fat?
|
dinitrophenol (DNP)
|
|
|
mechanism of dinitrophenol?
|
DNP is an uncoupler, it increases oxidation and decreases phosphorylation
|
|
|
most common cause of hyperthyroidism?
|
Grave's disease
|
|
|
what would be the effect of hypothyroidism on weight?
|
hypothyroidism would tend to cause increased weight gain
|
|
|
what condition is characterized by autoantibodies to the TSH receptor, resulting in high thyroid hormone with low TSH levels?
|
Grave's disease
|
|
|
how are the thyroid hormone levels abnormal in patient's with Grave's disease?
|
Thyroid hormone levels are high
TSH levels are low |
|
|
what enzyme is deficient in Von Gierke's disease?
|
Glucose-6-phosphatase
|
|
|
what enzyme is deficient in Pompe's disease?
|
lysosomal glucosidase
|
|
|
what is the normal function of lysosomal glucosidase?
|
lysosomal glucosidase normally breaks down glycogen in lysosomes
|
|
|
what enzyme is deficient in Forbe's/Cori's disease?
|
debranching enzyme
|
|
|
what are the 3 names for the disease is characterized by deficiency in the glycogen debranching enzyme?
|
Limit Dextrinosis = Forbe's disease = Cori's disease
|
|
|
what are the two names for the disease characterized by deficiency in the enzyme that adds branches to glycogen?
|
Amylopectinosis = Anderson's disease
|
|
|
what disease is caused by deficiency in muscle phosphorylase?
|
McArdle's syndrome
|
|
|
what is the deficient enzyme in McArdle's syndrome?
|
muscle phosphorylase
|
|
|
what enzyme is deficient in Hers' disease?
|
liver phosphorylase
|
|
|
what disorder is characterized by low blood lactate after exercise, and what causes this disorder?
|
McArdle's syndrome; caused by deficiency of muscle phosphorylase that would normally break down glycogen in muscle cells
|
|
|
what are the 6 glycogen storage diseases?
|
(1) Von Gierke's disease
(2) Pompe's disease (3) Cori's disease (4) Andersen's disease (5) McArdle's syndrome (6) Hers' disease |
|
|
what glycogen storage disease would resultin accumulation of glycogen in lysosomes?
|
Pompe's disease
|
|
|
what glycogen storage disease would result in fasting hypoglycemia and the accumulation of characteristic branched polysaccharides?
|
Cori's disease
|
|
|
what is the protein at the core of the glycogen molecule?
|
glycogenin
|
|
|
disease caused by deficiency in branching enzyme
|
Andersen's disease
|
|
|
what are the two glycogen storage diseases caused by glycogen phosphorylase deficiencies?
|
(1) McArdle's disease: muscle
(2) Hers' disease: liver |
|
|
what is the difference in manifestation of McArdle's disease and Hers' disease?
|
(1) McArdle's disease results in poor exercise tolerance
(2) Hers' disease results in hepatomegaly and hypoglycemia |
|
|
what is the normal function of glycogen phosphorylase?
|
sequential removal of glucose-1-phosphates from end of glycogen, until 4 residues are on either side of a branch point
|
|
|
what enzyme is used for synthesis of glucose from glycogen and is present in liver and kidneys, but not present in muscle cells?
|
glucose-6-phosphatase
|
|
|
what tissues have glucose-6-phosphatase?
|
liver and kidneys
|
|
|
what causes Celiac disease?
|
inability to digest and absorb gluten
|
|
|
what is the result of exposure to gluten in patients with Celiac disease?
|
damage to intestinal mucosa and inhibition of absorption of many nutrients
|
|
|
what are 3 sources of gluten?
|
(1) Wheat
(2) Rye (3) Barley |
|
|
In patients with Celiac disease, why do some symptoms appear gradually over time?
|
failure to absorb many vitamins and minerals leads to some symptoms that appear gradually over time
|
|
|
normally, what are serum transaminase levels?
|
very low
|
|
|
what are the expected serum transaminase levels in a patient with an acute liver disease?
|
ALT elevated more than AST
|
|
|
what are the expected serum transaminase levels in a patient with a chronic liver disease?
|
AST elevated more than ALT
|
|
|
what are the expected serum transaminase levels in a patient with an MI?
|
elevated AST only
|
|
|
why would a person deficient in B6 have abnormal EEG and seizures?
|
B6 deficiency would lead to decrease in GABA because the cofactor form of B6 is PLP, which is the cofactor for decarboxylation of glutamate to GABA. Low GABA reduces inhibitory effects of GABA, leading to abnormal EEG and seizures
|
|
|
what B vitamin is needed to form GABA?
|
B6 --> PLP --> cofactor for decarboxylation of glutamate to GABA
|
|
|
enzyme deficient in PKU?
|
phenylalanine hydroxylase
|
|
|
reaction catalyzed by Phe Hydroxylase?
|
Phe --> Tyr
|
|
|
what causes the neurological effects seen in patients with PKU?
|
Phe Hydroxylase deficiency leads to buildup of neurotoxic metabolites phenylacetate, phenylpyruvate, and phenyl lactate
|
|
|
4 roles of tyrosine that could be impaired in a patient with PKU?
|
(1) Catecholamines
(2) Thyroid Hormone (3) Melanin (4) Fumarate |
|
|
enzyme deficient in patient with Maple Syrup Urine Disease?
|
alpha-ketoacid dehydrogenase complex
|
|
|
two types of Maple Syrup Urine Deficiency
|
(1) Classic MSUD: deficiency of a-ketoacid DH complex
(2) Thiamin Responsive MSUD: decreased affinity of a-ketoacid DH complex for the TPP cofactor |
|
|
why does pyruvate carboxylase deficiency lead to hypoglycemia?
|
without pyruvate carboxylase, pyruvate is not converted to OAA for gluconeogenesis
|
|
|
what causes Fanconi-Bickel Syndrome?
|
abnormal GLUT2 receptor that prevents glucose from being exported from the liver following gluconeogenesis
|
|
|
what causes von Gierke's disease?
|
glucose-6-phosphatase deficiency, resulting in hypoglycemia and accumulation of glycogen
|
|
|
what causes Liddle's syndrome?
|
consitutive activating mutation in the Na+ channel that resporbs sodium in the renal tubular epithelial cells
|
|
|
what is the name of the disease that causes pseudohyperaldosteronism?
|
Liddle's syndrome
|
|
|
what disease is caused by constitutive activating mutation in the Na+ channel that resorbs sodium in the renal tubule epithelial cells?
|
Liddle's syndrome (pseudohyperaldosteronism)
|
|
|
what condition is caused by constitutively activating mineralocorticoid receptor mutatio?
|
Early-onset hypertension
|
|
|
what causes early-onset hypertension?
|
constitutively activating mutations to the mineralocorticoid receptor
|
|
|
why can progesterone function as partial agonist to mineralocorticoid receptor in early-onset hypertension?
|
the constitutively activating mineralocorticoid receptor mutations also alter the receptor's specificity of ligand binding, allowing progesterone to act as partial agonist
|
|
|
what is another name for Addison's disease?
|
adrenal hypocorticoidism
|
|
|
what is another name for adrenal hypocorticoidism?
|
Addison's disease
|
|
|
what are 7 signs/symptoms of Addison's disease?
|
(1) Adrenal Crisis
(2) Weakness (3) Anorexia (4) Weight Loss (5) Hypotension (6) Hypovolemia (7) Hyperpigmentation |
|
|
is hyperpigmentation associated with Addison's disease or Cushing's disease and why?
|
BOTH Addison's disease and Cushing's disease are associated with hyperpigmentation because both conditions can lead to high POMC expression (in order to generate more ACTH), which can increase melanocyte-stimulating hormone (MSH) levels
|
|
|
why might addison's disease cause hyperpigmentation?
|
* Addison's disease is a hypocorticoidism syndrome in which the adrenal glands make less cortisol.
* In response to low cortisol, the pituitary makes more ACTH, which is derived from POMC * POMC is also the precursor for melanocyte stimulating hormone (MSH) and therefore when POMC levels increase, so do MSH levels |
|
|
how can hypopigmentation differentiate primary from secondary hypocorticoidism?
|
* If hyperpigmentation is present, primary adrenal insufficiency is indicated
* This is because, in secondary adrenal insufficiency, the pituitary is not making and secreting ACTH from POMC * If POMC levels are not increasing to increase ACTH levels, then MSH (also from POMC) will not increase as well |
|
|
does primary or secondary adrenal insufficiency present with low ACTH levels?
|
low ACTH levels are seen in secondary hypocorticoidism
|
|
|
which type of hypocorticpoidism presents with normal or high ACTH levels?
|
primary adrenal insufficiency (primary hypocorticoidism)
|
|
|
diagram the algorithm for determination of adrenocortical insufficiency
|
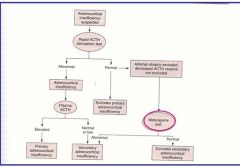
|
|
|
what test is used to determine ACTH reserve and how is it interpreted?
|
Metyrapone test
* Basis: Metyrapone prevents formation of cortisol from 11-deoxycortisol by blocking 11b-hydroxylase. If metyrapone is administered and ACTH levels INCREASE, primary * Interpretation: If metyrapone is administered and ACTH and CRH levels increase, secondary adrenal insufficiency can be ruled out (bc the hypothalamo-pituitary-adrenal axis is intact) |
|
|
what are the 2 ACTH resistance syndromes?
|
(1) Familial glucocorticoid deficiency
(2) Triple A (Allgrove) Syndrome |
|
|
what is familial glucocorticoid deficiency?
|
* ACTH resistance syndrome
* Hypoglucocorticoidism WITHOUT mineralocorticoid deficiency * Elevated ACTH due to melanocortin-2 receptor defect |
|
|
what disease results in hypoglucocorticoidism WITHOUT mineralocorticoid deficiency?
|
Familial glucocorticoid deficiency
|
|
|
what is the relationship between ACTH and melanocyte-stimulating hormone?
|
ACTH and MSH are both derived from POMC and therefore, conditions that elevate ACTH often also elevate MSH and result in hyperpigmentation (ie Addison's disease)
|
|
|
what ACTH resistance syndrome is caused by a defect in the melanocortin-2 receptor?
|
familial glucocorticoid deficiency
|
|
|
what is Allgrove syndrome?
|
* AKA Triple A syndrome = "Addison-like glucocorticoid deficiency, Achalasia, and Alacrimia"
* ACTH resistance Syndrome * Caused by defects in the AAAS gene that is highly expressed in adrenal cortex and CNS, and codes for a protein in the nuclear pore complex |
|
|
what causes Allgrove syndrome?
|
* Defect in AAAS gene that encodes a nuclear pore protein that plays a role in cytoplasmic trafficking and peroxisomal function
* AAAS gene is highly expressed in adrenal cortex and CNS |
|
|
what is the classic presentation of Triple A syndrome?
|
* Primary adrenal insufficiency
* Hypoglycemic seizures * Shock |
|
|
what does "Triple A" in "Triple A syndrome" stand for?
|
Achalasia-Addisonionism-Alacrimia
OR Achalasia - Adrenocortical insufficiency - Alacrimia |
|
|
what is alacrimia?
|
lack of tears
|
|
|
what is achalasia?
|
failure of smooth muscle fibers to relax, often referring specifically to failure of esophageal sphincter to relax (causing esophageal dysmotility)
|
|
|
what is the other name for congenital adrenal hyperplasia?
|
adrenogenital syndrome
|
|
|
what is congenital adrenal hyperplasia?
|
* Several autosomal recessive disorders
* Relative defect/deficiency in adrenal steroid synthesis, resulting in some degree of cortisol deficiency (+/- aldosterone deficiency or excess) * Elevated Androgens are present in some cases * Pseudohermaphroditism in females * NEARLY ALL ARE CAUSED BY: 21-beta-hydroxylase defect |
|
|
what family of conditions almost always results from deficiency in activity of 21b-hydroxylase activity?
|
congenital adrenal hyperplasia (adrenogenital syndrome)
|
|
|
what is 21b-hydroxylase?
|
* 21b-hydroxylase is a cytochrome p450 enzyme that is involved in the synthesis of the steroid hormones aldosterone and cortisol
* 21b-hydroxylase is a monooxygenase that hydroxylates carbon 21 in progesterone to form 11-deoxycorticosterone (aldosterone synthesis) and hydroxylates carbon 21 in 17a-hydroxyprogesterone to form 11-deoxycortisol (cortisol synthesis) |
|
|
what does 21b-hydroxylase do in cortisol synthesis?
|
hydroxylates 17a-hydroxyprogesterone to form 11-deoxycortisol (penultimate step)
|
|
|
what does 21b-hydroxylase do in aldosterone synthesis?
|
hydroxylates progesterone to form 11-deoxycorticosterone
|
|
|
11b-hydroxylase is involved in the synthesis of what steroid hormone?
|
cortisol
|
|
|
what does 11b-hydroxylase do in cortisol synthesis?
|
11b-hydroxylase catalyzes conversion of 11-deoxycortisol to cortisol
|
|
|
21b-hydroxylase is involved in the synthesis of what hormones?
|
aldosterone and cortisol
|
|
|
what is the treatment for congenital adrenal hyperplasia and what is the negative side effect?
|
Fludrocortisone (glucocorticoid and mineralocorticoid) administered during pregnancy can decrease/prevent virilization of an affected female fetus, but can affect brain development
|
|
|
defect in which enzyme can lead to females presenting with pseudohermaphroditism?
|
21-beta-hydroxylase (congenital adrenal hyperplasia)
|
|
|
what is the other name for adrenal hypercorticoidism?
|
Cushing's syndrome
|
|
|
what is the other name for Cushing's syndrome?
|
Adrenal hypercorticoidism
|
|
|
what is the hormonal difference between Cushing's syndrome and Addison's disease?
|
Addison's = HYPOcorticoidism
Cushing's = HYPERcorticoidism |
|
|
what are the complications and symptoms of Cushing's disease?
|
"CUSHINGOID"
* Cataracts (due to high cortisol) * Ulcers (think of this as "stress = cortisol" and you know that stress can increase ulcer formation) * Skin: striae (due to abdominal fat gain), thin skin, bruising * Hypertension, Hirsutism (excessive hair), Hyperglycemia, and hyperpigmentation * Infections * Necrosis (avascular) * Glycosuria * Osteoporosis, Obesity * Immunosuppression, insomnia * Diabetes, dysmenorrhea (and impotence), depression |
|
|
what condition can result in "moon face" due to accumulation of facial fat and increased water retention?
|
Cushing's syndromes
|
|
|
what is the goal of pharmocological treatments for Cushing's syndromes?
|
pharmacological treatments for Cushing's usually try to LOWER CORTISOL SYNTHESIS, often by inhibiting ACTH secretion
|
|
|
what receptors are targeted by medications for Cushing's disease that reduce ACTH secretion?
|
adenoma-specific somatostatin-5 receptors
|
|
|
what disease is treated with somatostatin analogs that bind adenoma-specific somatostatin-5 receptors?
|
Cushing's
|
|
|
what are 6 forms of adrenocorticoid dysfunction?
|
(1) Addison's disease
(2) Cushing's disease (3) Adrenal adenoma or carcinoma (4) Ectopic ACTH (5) Ectopic CRH (6) Iatrogenic Cushing's syndrome |
|
|
diagram the 6 forms of adrenocorticoid dysfunction
|

|
|
|
what is Nelson's syndrome?
|
* ACTH-secreting adenoma that forms or enlarges after a bilateral adrenalectomy (biADx)
|
|
|
what is the presentation of a patient with Nelson's syndrome?
|
* Hyperpigmentation
* Manifestations of an expanding intrasellar mass lesion (visual defects, headache) |
|
|
What is Conn's syndrome and its two causes?
|
* Primary hyperaldosteronism
* Caused by uni-lateral adrenocortical adenoma (~73%) * Caused by bilateral cortical hyperplasia (~27%) |
|
|
what is primary hyperaldosteronism?
|
hypersecretion of aldosterone by adrenal glands WITHOUT excessive renin secretion by the kidneys
|
|
|
what is the presentation of a patient with Conn's disease?
|
* Hypertension due to excess aldosterone (Na+ and H2O retension)
* Hyperkalemia and resultant muscle weakness ( due to increased K+ excretion) * Low renin levels (negative feedback by aldosterone on kidneys) |
|
|
what are the treatments for Conn's syndrome?
|
(1) Laparoscopic adrenalectomy
(2) Lifelong spironolactone therapy |
|
|
what is spironolactone?
|
aldosterone antagonist (and therefore diuretic) that can be used to treat Conn's syndrome
|
|
|
what is a pheochromocytoma?
|
a site of ectopic adrenal tissue (ie. neuroendocrine tumor of chromaffin cells of adrenal medulla) that hypersecretes catecholamines (epi and norepi)
|
|
|
what is the clinical presentation of a patient with a pheochromocytoma?
|
Signs/symptoms are those of excessive sympathetic activity:
* Eposodic or sustained hypertension * Sweating * Palpitations * Hyperglycemia * Glycosuria |
|
|
what is the epidemiology of pheochromocytoma?
|
* Adults of all ages, especially between 30-50 years
* Both sexes |
|
|
what is a pheochromocytoma called when it is outside of the adrenal glands?
|
paraganglioma
|
|
|
why are pheochromocytomas called the "10% tumor"?
|
* 10% are malignant
* 10% are bilateral * 10% are ectopic (not in an adrenal medulla) * 10% occur in children * 10% are familial * 10% recur within 10 years * 10% are associated with MEN II * 10% present after a stroke |
|
|
what percent of pheochromocytoma present following a stroke?
|
10%
|
|
|
what percent of pheochromocytoma are familial?
|
10%
|
|
|
what percent of pheochromocytoma occur in children?
|
10%
|
|
|
what percent of pheochromocytoma are associated with MEN II?
|
10%
|
|
|
what is the preferred treatment for pheochromocytoma?
|
laparoscopic surgery
|
|
|
what are the pharmacotherapies for pheochromocytoma?
|
* Alpha-1-adrenergic antagonist
* Catecholamine synthesis inhibitors |
|
|
what is the classic disease that reduces diffusion capacity and thus may produce an A-a gradient and cause hypoxemia?
|
fibrosis
|
|
|
below what Hb saturation does cyanosis develop?
|
Hb sat below 75% produces the bluish tint of cyanosis
|
|
|
how would an increased temperature affect the affinity of Hb for O2?
|
increased temperature DECREASES affinity of Hb for O2 (right shift)
|
|
|
how do left and right shifts of the hemoglobin-O2 dissociation curve reflect changes in affinity of Hb for O2?
|
* Right shifts ==> decreased affinity of Hb for O2
* Left shifts ==> increased affinity of Hb for O2 |
|
|
what is the immediate treatment for patient with carbon monoxide poisoning?
|
100% oxygen
|
|
|
what is Hb called when it is bound to carbon monoxide?
|
carboxyhemoglobin
|
|
|
what is carboxyhemoglobin?
|
Hb bound to CO
|
|
|
how and why is the O2 carrying capacity of Hb changed in patients with carbon monoxide poisoning?
|
heme groups bound to CO can not bind O2, and therefore the O2 carrying capacity is reduced by carboxyhemoglobin
|
|
|
what A-a gradient would you expect for a patient on 100% oxygen?
|
60 mmHg
|
|
|
when a patient is on 100% oxygen (at sea level), what is the PaO2?
|
600 mmHg
|
|
|
when a patient is on 100% oxygen (at sea level), what is the PAO2?
|
660 mmHg
|
|
|
what is the half life of CO in CO poisoning?
|
4 hours
|
|
|
how and why does 100% oxygen change the half life of CO in patients with carbon monoxide poisoning?
|
100% O2 competitively inhibits CO and decreases the half life of CO from 4 hours to less than 1 hour
|
|
|
what is the normal concentration of oxygen dissolved in plasma?
|
0.3 mL/dL
|
|
|
how does placing a patient on 100% oxygen change the O2 content of that patient's blood?
|
100% oxygen increases the O2 content from about 20 mL/dL to about 22 mL/dL (increased by 2 mL/dL)
|
|
|
on room air, what is the normal PaO2?
|
90 mmHg
|
|
|
at 3 atm of pressure (hyperbaric chamber), to what levels can the PaO2 and dissolved oxygen be raised?
|
At 3 atm, PaO2 can be increased to about 1800 mmHg and the dissolved oxygen can be increased to 5-6 mL/dL
|
|
|
how and why would excess mineralocorticoid affect carbon dioxide levels in venous blood?
|
* Mineralocorticoid excess would INCREASE venous CO2 levels
* This is because the mineralocorticoid would increase tubular H+ secretion in kidneys, causing an increased resorption of HCO3- into the blood (also causing metabolic alkalosis) |
|
|
what is bronchiectasis?
|
irreversible upper airway dilation
|
|
|
what condition is defined as irreversible upper airway dilation?
|
Bronchiectasis
|
|
|
what are 3 causes of bronchiectasis?
|
(1) Kartagener's syndrome
(2) Cystic fibrosis (3) Idiopathic |
|
|
what is the most common clinical presentation of bronchiectasis?
|
chronic cough producing mucopurulent discharge
|
|
|
is bronchiectasis an obstructive or restrictive pulmonary disesae, and why?
|
Bronchiectasis is obstructive because chronic infections produce cycle of airway damage and destruction
|
|
|
what condition is defined as the complete mirror image reversal of the thoracic and abdominal organs?
|
situs inversus
|
|
|
what percent of patients with situs inversus also have Kartagener's syndrome?
|
20%
|
|
|
with respect to cystic fibrosis, what does CTFR stand for?
|
Cystic fibrosis Transmembrane Conductance Regulator
|
|
|
what percent of bronchiectasis cases can be accounted for by cystic fibrosis?
|
CF accounts for 50% of bronchiectasis cases
|
|
|
what condition is tested for by the "sweat chloride test"?
|
cystic fibrosis
|
|
|
what is salmeterol?
|
beta-2 adrenergic agonist used as a bronchodilator
|
|
|
what is albuterol?
|
beta-2 adrenergic receptor agonist used as bronchodilator
|
|
|
what is ipatropium?
|
muscarinic (M3) receptor antagonist used as bronchodilator
|
|
|
what is tiotropium?
|
muscarinic (M3) receptor antagonist used as bronchodilator
|
|
|
what type of pulmonary disease increases airway resistance and makes it difficult for patients to exhale?
|
obstructive disease
|
|
|
what type of pulmonary disease decreases lung compliance and makes it difficult for patients to inspire?
|
restrictive disease
|
|
|
how does restrictive pulmonary disease affect residual volume?
|
restrictive disease DECREASE residual volume
* RV decreases because compliance decreases, making it "easier" to deflate lungs and establishing lung-chest equilibrium point at a lower lung volume |
|
|
how does obstructive pulmonary disease affect residual volume?
|
obstructive diseases INCREASE residual volume
* RV increases because elasticity decreases, making it more difficult to deflate lungs and establishing lung-chest equilibrium point at a higher lung volume |
|
|
what two conditions are generally involved in COPD?
|
bronchitis and emphysema
|
|
|
what condition is associated with "pink puffer" presentation?
|
emphysema
|
|
|
do patients with emphysema have an increased or decreased resting lung volume?
|
increased
|
|
|
what condition is associated with "blue-bloater" presentation?
|
bronchitis
|
|
|
in patients with bronchitis, hypertrophy and inflammation in which part of the respiratory system is responsible for the increased airway resistance and reduced alveolar ventilation?
|
bronchioles
|
|
|
is interstitial lung disease restrictive or obstructive?
|
restrictive
|
|
|
when are testosterone levels high in a normal male?
|
in utero and shortly after birth (between puberty and senescence)
|
|
|
what are the signs/symptoms of Kartagener's syndrome?
|
* Asthenoxoospermia (decreased sperm motility)
* Multiple sinus and pulmonary infections * Chronic sinusitis * Bronchiectasis |
|
|
what causes Kartagener's syndrome?
|
congenital absence of dynein
|
|
|
what stain is used to identify dead sperm?
|
eosin vital stain
|
|
|
what is necrozoospermia?
|
dead sperm
|
|
|
what is asthenozoospermia?
|
reduced sperm motility
|
|
|
what is the normal intratesticular temperature?
|
about 34*C
|
|
|
what is a varicocele?
|
* Abnormal enlargement of pampiniform venous plexus in the scrotum
* Occurs more often on the left side, where the pampiniform plexus drains to the left renal vein |
|
|
on which side is testicular varicocele most common and why?
|
left side, because the left pampiniform plexus drains into the left renal vein (right pampiniform plexus drains to IVC)
|
|
|
what is the relation between varicocele and oligozoospermia?
|
varicocele is palpable in about 40% of men with oligozoospermia
|
|
|
what is oligozoospermia?
|
low sperm count
|
|
|
what is the other name for androgen insensitivity syndrome?
|
testicular feminization syndrome
|
|
|
how is androgen insensitivity syndrome inherited?
|
X-linked recessive
|
|
|
what is androgen insensitivity syndrome?
|
* AKA testicular feminization syndrome
* X-linked recessive * Failure of normal masculinization of external genitalia in chromosomally male (46, XY) individuals * Depending on residual function of the androgen receptor, the condition can be complete or partial (CAIS or PAIS) |
|
|
what differentiates androgen insensitivity syndrome from 5-alpha-reductase deficiency?
|
* Individuals with androgen insensitivity syndrome have normal testes with normal production of testosterone and normal conversion of testosterone to DHT
* 5-alpha reductase normally converts testosterone to DHT and therefore individuals with 5a-reductase deficiency have abnormal testosterone and DHT levels |
|
|
why dont patients with androgen insensitivity syndrome develop fallopian tubes, a uterus, or a proximal vagina?
|
Patients with AIS do not develop these organs because the testis still produce normal amounts of anti-mullerian hormone
|
|
|
what are the 6 categories of causes of impotence?
|
(1) Neurogenic
(2) Endocrinologic (3) Psychogenic (4) Vasculogenic (5) Drug-induced (6) Miscellaneous |
|
|
compare the pressure-volume curves for normal lungs and for diseased lungs (emphysema and fibrosis)
|

|
|
|
what are the key features of infant respiratory distress syndrome?
|
(1) Decreased compliance
(2) Atelectasis (lung collapse) (3) Increased inspiratory work |
|
|
what is the treatment for infant respiratory distress syndrome?
|
* Corticosteroid administration through ET tube
* Positive pressure ventilation with supplemental oxygen |
|
|
why must supplemental oxygen be limited in patients with infant respiratory distress syndrome?
|
supplemental oxygen can lead to toxic oxygen damage to retina and lungs
|
|
|
what leads to the formation of hyaline membranes in patients with infant respiratory distress syndrome?
|
fibrin-rich exudates are common in alveolar space of affected patients, and these exudates lead to the formation of hyaline membranes
|
|
|
what characterizes acute respiratory distress syndrome?
|
(1) Pulmonary edema
(2) Stiff lungs (3) Hypoxemia |
|
|
what causes infant respiratory distress syndrome?
|
reduced or absent surfactant production
|
|
|
what causes acute respiratory distress syndrome?
|
microbial endotoxins leading to inflammatory damage to all capillaries, including pulmonary capillaries
|
|
|
in patients with ARDS, how and why is pulmonary capillary permeability changed?
|
pulmonary capillary permeability increases as a result of inflammatory damage to the capillaries resulting from microbial endotoxins
|
|
|
what type of pulmonary edema is present in patients with ARDS?
|
non-hydrostatic pulmonary edema (edema due to capillary permeability rather than increased hydrostatic pressure)
|
|
|
what causes hyaline membrane formation in patients with ARDS?
|
death of type I pneumocytes results in formation of hyaline membrane
|
|
|
why is the alveolar barrier thickened in patients with ARDS?
|
death of type I pneumocytes results in formation of hyaline memrane (fibrin) formation that increases the thickness of the alveolar membrane (reducing rate of gas exchange)
|
|
|
what is heliox?
|
helium-oxygen mixture with reduced viscosity that is used to treat asthmatic attacks
|
|
|
in both health and disease, when is airway resistance maximal?
|
as lung volume decreases (expiration)
|
|
|
3 major components of asthma?
|
(1) Paroxysmal narrowing of bronchial airways
(2) Airway inflammation (3) Hyper-responsiveness |
|
|
what are the common triggers of asthma attack?
|
* Allergic antigens
* Infection * Exercise * Emotional stress * Cold and dry air * Night |
|
|
what is methacholine?
|
nonselective muscarinic receptor agonist used as a test for asthma (bronchial provocation test)
|
|
|
when is an extra-thoracic obstruction moved into the airways?
|
extra-thoracic obstructions are pulled into airways by negative pressure DURING INSPIRATION
|
|
|
when is n intra-thoracic obstruction moved into airways?
|
intra-thoracic obstructions are pushed into airways by positive pressure during exhalation
|
|
|
when would a tracheal tumor result in difficulty breathing and why?
|
* Tracheal tumors are intra-thoracic obstructions and would therefore cause the MOST DIFFICULTY DURING EXHALATION
* Positive pressure during exhalation would push the mass into the airways |
|
|
when would laryngeal edema cause the most difficulty in breathing?
|
* Laryngeal edema is an extra-thoracic obstruction and would therefore cause the most difficulty DURING INSPIRATION when the negative pressure would pull the obstruction into airways
|
|
|
what techniques are used to measure residual volume?
|
(1) Helium dilution
(2) Box plethysmography |
|
|
how is diffusion capacity affected by emphysema, fibrosis, and anemia?
|
diffusion capacity is decreased in emphysema, fibrosis, and anemia
|
|
|
how is diffusion capacity affected by asthma and bronchitis?
|
diffusion capacity may be normal in patients with bronchitis and asthma
|
|
|
what is the purpose of cardiopulmonary exercise testing?
|
evaluates dyspnea and identifies exercise-induced asthma
|
|
|
what is the home test for self-evaluation of airway narrowing during asthma attack?
|
Peak Expiratory Flow Rate measurement
|
|
|
what is done in the maximal voluntary ventilation (MVV 12 sec) test?
|
patient breaths as fast and deep as possible for 12 seconds to test for muscle strength and endurance (and volume of air moved)
|
|
|
what causes a congenital iodide transport defect?
|
Mutations in the NIS gene
|
|
|
how is congenital iodide transport defect inherited?
|
autosomal recessive
|
|
|
what characterizes congenital iodide transport defects due to NIS gene mutations?
|
* Hypothyroidism
* Goiter * Low thyroid iodide uptake * Low saliva/plasma iodide ratio (iodide is high in plasma but low in saliva) |
|
|
why is the salive/plasma iodide ratio decreased in patients with NIS gene mutations?
|
* NIS gene is expressed in thyroid, salivary glands, gastric mucosa, and the lactating mammary gland.
* If the NIS gene is mutated, transport of iodide out of the plasma (and into salivary glands) will be decreased. Plasma levels will remain high. |
|
|
what causes Pendred syndrome?
|
mutations in the pendrin gene (PDS)
|
|
|
what characterizes Pendred syndrome?
|
* Sensorineural deafness
* Goiter (occasionally) * Impaired iodide organification (incorporation of iodide into thyroid hormone) |
|
|
what is the Wolff-Chaikoff effect?
|
* Reduction of thyroid hormone synthesis after large amounts of inorganic iodide are administered.
* Possibly due to down-regulation of NIS * Lasts about 10 days |
|
|
when is the Wolff-Chaikoff effect clinically useful?
|
useful in treating some forms of hyperthyroidism
|
|
|
what causes Allan-Herndon-Dudley syndrome?
|
* Condition of X-linked mental retardation and hypotonia that results from mutations to the SLC16A2 gene that encodes thyroid transporter protein on target cells
|
|
|
what condition is caused by mutations in the gene that codes for the protein that transports thyroid hormone into target cells?
|
Allan-Herndon-Dudley syndrome
|
|
|
what characterizes "thyroid storm"?
|
(1) Tachycardia
(2) Restlessness (3) Sweating |
|
|
what is the other name for Plummer syndrome?
|
toxic multinodular goiter
|
|
|
what causes Plummer syndrome?
|
* A functioning follicular adenoma hypersecreting T3/T4 to cause hyperthyroidism
* Commonly due to TSH-R mutation |
|
|
what condition results from a functioning follicular adenoma that hypersecretes T3/T4 (causing hyperthyroidism)?
|
Plummer syndrome
|
|
|
what is the treatment for Plummer Syndrome?
|
antithyroid drugs such as propylthiouracil or methimazole, followed by treatment with oral radioactive iodine or unilateral lobectomy
|
|
|
what is propylthiouracil?
|
antithyroid drug
|
|
|
what is methimazole?
|
antithyroid drug
|
|
|
what disease is caused by aberrant physiological activation of the TSH receptor by TSH-R autoantibodies?
|
Grave's disease
|
|
|
what causes gestational trophoblastic disease?
|
in molar pregnancy and choriocarcinoma, excess levels of hCG can activate the TSH-R
|
|
|
how is congenital hyperthyroidism inherited?
|
autosomal dominant inheritance
|
|
|
what causes congenital hyperthyroidism?
|
autosomal dominant gain-of-function mutation in the TSH-R
|
|
|
what causes TSH resistance?
|
autosomal recessive loss-of-function mutation in the TSH-R
|
|
|
what is the presentation of TSH resistance?
|
* Thyroid hypoplasia
* Normal to low free T4 * Elevated TSH |
|
|
what condition is characterized by inflammation of thyroid tissue that causes leakage of TH into circulation?
|
acute thyroiditis
|
|
|
why does acute thyroiditis typically resolve spontaneously after several weeks?
|
exhaustion of TH stores
|
|
|
what is the most common form of hypothyroidism?
|
Hashimoto's thyroiditis
|
|
|
what causes Hashimoto's thyroiditis?
|
* Autoantibodies against several thyroid components, leading to destructive inflamation of the thyroid gland
|
|
|
what is the presentation of a patient with Hashimoto's thyroiditis?
|
* Hypothyroidism
* Myxedema * Cold intolerance * Thick, dry skin * Hair loss * Muscle weakness * Lethargy * Apathy * Goiter is possible |
|
|
what is myxedema?
|
* Deposition of mucopolysaccharides in the dermis, resulting in swelling of the affected area (often the face)
* Common in hypothyroidism |
|
|
what gender is affected more frequently by autoimmune thyroid disease?
|
AITD is 5-10 times more prevalent in women than in men
|
|
|
what is the growth and development abnormality caused by hypothyroidism?
|
cretinism
|
|
|
good comparison of hypo and hyper thyroidism (just view the comparison)
|
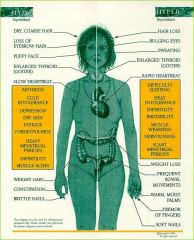
|
|
|
how are multiple endocrine neoplasias inherited?
|
autosomal dominant inheritance
|
|
|
what is Wermer's syndrome and its key features?
|
* Multiple endocrine neoplasia type 1 (MEN 1)
* Primary hyperParathyroidism * Pancreatic tumors * Pituitary tumors |
|
|
What is Sipple Syndrome and its key features?
|
* Multiple endocrine neoplasia type 2a (MEN 2a)
* Medullary carcinoma of the thyroid * Pheochromocytoma * Primary hyperparathyroidism * Hirschsprung's megacolon |
|
|
what type of MEN syndrome presents is associated with pheochromocytoma?
|
MEN 2a = Sipple Syndrome
MEN 2b syndrome |
|
|
what type of MEN syndrome almost invariably involves primary hyperparathyroidism?
|
MEN 1 = Wermer's syndrome
|
|
|
what mutation leads to MEN 1?
|
mutation in MENIN tumor suppressor gene
|
|
|
what mutation leads to MEN 2a syndrome?
|
mutation in RET gene
|
|
|
what mutation leads to Sipple syndrome?
|
mutation in RET gene (an oncogene)
|
|
|
what mutation leads to Wermer's syndrome?
|
mutation in MENIN tumor suppressor gene
|
|
|
mutations in what gene lead to MEN conditions that commonly involve pheochromocytoma?
|
RET gene (oncogene)
|
|
|
what type of MEN syndromes are treated with surgical removal of adrenal medullary tumors?
|
Adrenal medullary tumors are present in MEN 2a and MEN 2b syndromes, and are treated with surgical removal
|
|
|
what pulmonary condition can result from diffuse hypoxic vasoconstriction?
|
pulmonary hypertension
|
|
|
what are the signs and symptoms of pulmonary hypertension?
|
(1) Fatigue and exertional syncope
(2) Pulmonic valve regurgitation (3) RV hypertrophy (4) Signs of Cor pulmonale (pulmonary heart disease) |
|
|
what are the signs of Cor pulmonale?
|
(1) RV systolic heart failure
(2) RV dilation (3) Dyspnea (4) Effort-related syncope (5) JVD (a-waves) (6) Abdominal distension and ascites (7) Peripheral edema |
|
|
5 causes of hypoxemia
|
(1) High Altitude
(2) Hypoventilation (3) Fibrosis (4) V/Q defect (5) R-->L shunt |
|
|
where is tuberculosis most likely to present on a chest radiograph and why?
|
* Most likely to present in upper lobes because TB is caused by aerobic mycobacteria and the upper lobes have the highest alveolar oxygen partial pressures
|
|
|
what type of shunt is present if ventilation is zero?
|
If ventilation is zero, venous blood is not exposed to alveolar air and a RIGHT TO LEFT shunt is present (a shunt in which deoxygenated blood returns to systemic circulation)
|
|
|
what defect produces a venous admixture?
|
stenosis/narrowing of an airway
|
|
|
what defect produces a shunt?
|
complete obstruction of an airway
|
|
|
what defect produces dead-space ventilation?
|
a complete pulmonary embolism
|
|
|
what defect produces wasted ventilation?
|
stenosis/narrowing of a pulmonary artery or capillary
|
|
|
how does a pulmonary embolism affect CO2 delivery to alveoli?
|
pulmonary embolism reduces CO2 delivery to alveoli
|
|
|
what is the most common pulmonary perfusion disorder?
|
pulmonary embolism
|
|
|
what gives rise to most pulmonary emboli?
|
deep venous thrombosis
|
|
|
what is Virchow's triad?
|
* Three factors contributing to thromboses/emboli:
(1) Venous trauma (2) Stasis (3) Hypercoaguable state |
|
|
does pulmonary embolism result in hypoxemia?
|
yes
|
|
|
what is the initial result of a bronchial obstruction?
|
bronchial obstruction initially causes a right to left shunt, as regional hypoxia redirects perfusion
|
|
|
what type of shunt causes hypoxemia?
|
right to left shunts cause hypoxemia
|
|
|
why do left to right shunts NOT cause hypoxemia?
|
left to right shunts redirect blood from systemic to pulmonary circulation and do not deliver deoxygenated blood to systemic circulation
|
|
|
how can anatomic left to right shunt be diagnosed with Swan-Ganz catheter?
|
* A Swan cath in the right side of the heart can take a blood sample to measure oxygen saturation.
* Elevated Hb sat (above 75%) in the right heart indicates a L-->R shunt |
|
|
condition in which there is reversal of a L-->R shunt
|
Eisenmenger syndrome
|
|
|
how is Eisenmenger's syndrome diagnosed?
|
* 100% oxygen is administered and an arterial blood gas is taken after 20 minutes
* PaO2 should be about 600 mmHg when patient is on 100% O2 * The lower the PaO2 when on 100% O2, the more severe the right to left shunt |
|
|
what is the effect of 100% oxygen on hypoxemia caused by V/Q mismatch (partial bronchial obstruction) and why?
|
* 100% Oxygen corrects hypoxemia caused by V/Q mismatch (venous admixture)
* The increased PIO2 gets more oxygen past the partially obstructed airway to ventilate the downstream alveoli |
|
|
what is the affect of 100% oxygen on hypoxemia caused by R-L shunting (complete bronchial obstruction) and why?
|
* 100% oxygen does not correct the hypoxemia caused by R-L shunt
* The complete obstruction does not allow any inspired air, regardless of the PO2, ventilate the alveoli downstream from the obstruction |
|
|
diagram the algorithm for diagnostic approach to patient with hypoxemia
|
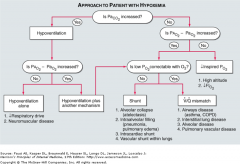
|

