![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
167 Cards in this Set
- Front
- Back
|
ions that determine acid-base balance
|
the concentrations of hydrogen and bicarbonate ions
|
|
|
why must the concentration of hydrogen and bicarbonate ions in the plasma be precise?
|
in order to:
-optimize enzyme activity -hemoglobin saturation of oxygen -myocardial contractility -rates of chemical reactions within cells |
|
|
hydrogen ion production
|
-they are continuously being produced as substrates and oxidized during the production of ATP, and therefore must be eliminated continuously by the kidneys and lungs
|
|
|
normal [H+] at 37C in arterial blood and extracellular fluid
|

35-45 nmol/L
which is equivalent to pH 7.45-7.35 |
|
|
normal plasam bicarbonate ion concentration
|
24 +/- 2 mEq/L
|
|
|
venous blood pH
|
-tends to be slightly more acidic than arterial
-normal pH 7.3-7.4 |
|
|
intracellular hydrogen ion concentration
|
160 nmol/L, equivalent to pH 6.8
|
|
|
acidosis and alkalosis terminology
|
-of course refer to disorders where the blood is overly acidic or alkaline
-but, a person with either disorder can have a normal pHa due to renal or pulmonary compensatory responses, or both -also, patients can have mixed disorders with both acidosis and alkalosis, in which case the more dominant disorder will dictate the pHa |
|
|
acidemia definition
|
pHa < 7.35
|
|
|
alkalemia definition
|
pH >7.45
|
|
|
what does base excess refer to?
|
the nonrespiratory, or metabolic, component of an acid-base disturbance
|
|
|
base excess when determined experimentally
|
-the amount of strong acid (hydrochloric acid for base excess >0) or strong base (sodium hydroxide for base excess <0) titrated to normalize the pHa of a blood sample under standard conditions of 37C, PaCO2 of 40 mmHg
|
|
|
base excess in practice
|
-is estimated by blood gas machines using algorithms based on the measured pHa, PaCO2, PaO2, and Hb
|
|
|
what is the normal value of base excess?
|
0
|
|
|
what does a base excess signifiacntly less than 0 mean?
|
(negative)
-the presence of a metabolic acidosis |
|
|
a value significantly greater than 0?
|
(positive)
-the presence of metabolic alkalosis |
|
|
3 systems that prevent changes in pHa
|
1. buffer systems
2. the ventilatory response 3. the renal response |
|
|
actions of these 3 systems
|
-when there is the introduction of small amounts of acids or bases the buffer systems are the first line of defense, and when the buffer system is overwhelmed and pHa deviates from normal, ventilatory responses first provide restoration in minutes, and later renal responses in hours to days
|
|
|
buffer system composition
|
-made of a base and its weak conjugate acid (for example bicarbonate and carbonic acid)
-the base binds excess acid, and the weak acid protonates base molecules, acting to maintain a constant pHa |
|
|
pKa
|
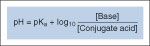
-is derived from the classic Henderson-Hasselbalch equation, it indicates the strength of an acid
-is the pH at which an acid is 50% protonated and 50% deprotonated |
|
|
what does a smaller pKa indicate?
|
a stronger acid
|
|
|
buffer systems in the body, in order of importance
|
1. bicarbonate buffer system
2. hemoglobin buffer system 3. other protein buffer systems 4. phosphate buffer system |
|
|
when does a biffer system function best?
|
at a pH near its pKa
|
|
|
5 components of the bicarbonate buffer system
|
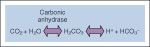
1. carbon dioxide
2. water 3. carbonic anhydrase 4. carbonic acid 5. bicarbonate -carbon dioxide is made by aerobic metabolism, it then reacts with water to form carbonic acid, which quickly and spontaneously deprotonates to form bicarbonate |
|
|
carbonic acid formation from carbon dioxide
|
-is a slow process
-however, the enzyme carbonic anhydrase is found in endothelium, erythrocytes, and the kidneys, and it catalyzes this reaction |
|
|
carbon dioxide and cell membranes
|
it permeats freely through cell membranes
|
|
|
bicarbonate system pKa
|
-has a pKa of 6.1
-this is very different from physiologic pH (and remember that buffer systems work best at a pH near their pKa) -however, the high concentration of bicarbonate ions in the blood, and the renal and pulmonary regulation of bicarbonate and carbon dioxide levels makes it the most important buffering system |
|
|
what is the 2nd most important buffer system?
|
the Hb buffer system
|
|
|
the hemoglobin buffer system
|
-RBCs are obviously packed with Hb, which has multiple proteinatable sites created by the imidazole side chains of histidine residues (pKa 6.8)
-because Hb is only in RBCs, its ability to buffer depends of bicarbonate -CO2 freely diffuses down a concentration gradient into RBCs and combines with water and carbonic anhydrase, forming carbonic acid, which rapidly deprotonates, with the formed proteins combining with Hb, and the bicarbonate anions exchanged electroneutrally back into plasma with Cl- ions ("chloride shift"); this allows the plasma bicarbonate pool to make use of intracellular carbonic anhydrase |
|
|
where does the reverse of this process take place?
|
-in pulmonary capillaries
-the carbon dioxide returns to the plasma and the lung alveoli for ventilatory elimination, allowing a large portion of extrapulmonary CO2 to be transported back to the lungs as plasma bicarbonate |
|
|
how else is CO2 transported?
|
by combining with Hb to form carbaminohemoglobin
|
|
|
Hb and CO2 affinity
|
-deoxyhemoglobin has a higher affinity for CO2 (Haldane effect), which facilitates removal of CO2 from peripheral tissues and release of it into the lungs
|
|
|
sensors of changes in CO2 concentration
|
-chemoreceptors peripherally in the carotid bodies and (to a lesser extent) aortic arches, and centrally in the ventral surface of the medulla oblongata of the brainstem respond in minutes to changes in CO2 or pHa by increasing or decreasing minute ventilation
|
|
|
relationship between PaCO2 and alveolar minute ventilation
|

is an inverse relationship
|
|
|
carotid bodies
|
-peripheral chemosensing organs at the bifurcation of the bilateral carotid arteries in the neck
|
|
|
in what nerve do the afferents from the carotid bodies to the CNS travel?
|
via the glossopharyngeal nerve
|
|
|
stimulus from peripheral and central chemoreceptors as pHa changes
|
-as the pHa approaches 7.4 the stimulus from these chemoreceptors to increase or decrease alveolar ventilation diminishes, making it impossible to completely correct or overcorrect
|
|
|
what percentage does the central chemoreceptor account for?
|
85%
|
|
|
response after bilateral endarterectomy
|
-it unintentionally ablates the carotid body organ
-patients will have no hypoxic ventilatory drive, only a slightly elevated resting PaCO2, and a blunted ventilatory response to increased PaCO2 |
|
|
renal regulation of bicarbonate
|
-the proximal tubules regulate bicarbonate levels by nearly completely reabsorbing bicarbonate from the glomerular filtrate, and by secretion of hydrogen ions inot the tubular lumen with concominant intracellular bicarbonate production
-under normal conditions, urine is free of bicarbonate due to proximal tubular reabsorption: secreted hydrogen ions assisted by luminal membrane bound carbonic anhydrase convert virtually all of the filtered bicarb into CO2 for reabsorption |
|
|
what happens to additional secreted hydrogen ions which dont take part in this system?
|
they are trapped b y water-soluble urinary buffers, phosphate and ammonia, for excretion in the urine
|
|
|
regulation of the renal buffering system
|
is by multiple things:
-PaCO2 -bicarbonate levels -urinary ammonia production |
|
|
what will a defect in proximal tubulae bicarbonate absorption result in?
|
bucarbonate-wasting disease (proximal renal tubular acidosis)
|
|
|
drug induced imparment of bicarbonate reabsorption
|
-can be caused by acetazolamide, a carbonic anhydrase inhibitor, which will impair bicarb absorption and production in the proximal tubules and cause a normal-anion gap metabolic acidosis
|
|
|
what is this drug used for?
|
to reverse iatrogenic chloride-resistant metabolic alkalosis
|
|
|
categorization of acid-base disturbances
|
as either respiratory or metabolic acidosis (<7.35) or alkalosis (>7.45)
|
|
|
how can they be further categorized?
|
as acute vs. chronic based on the duration of the disturbance, which is gauged clinically by the compensatory responses
|
|
|
stepwise approach to interpreting acid-base status
|
-is the pH value life-threatening (<7.1 requires immediate intervention)?
-does the value reflect acidosis or alkalosis? -could an acute increased in PaCO2 explain the entire blood gas picture? -is there evidence of a chronic respiratory disturbance? -is there evidence of an acute metabolic disturbance? -are compensatory changes present? -does the clinical history fit the acid-base picture? |
|
|
what is the most important adverse response to acidosis and alkalosis?
|
-depression of myocardial contractility (acidosis causes more dramatic effect on this than alkalosis)
|
|
|
acidemia and depression of myocardial contractility
|

-little effect occurs until pHa falls below 7.2, which is because acidemia induces the release of catecholamines, which helps to offset the decrease in myocardial contractility
-below 7.2, however, myocardial responsiveness to catecholamines diminishes and so does this compensatory response, so there is significant myocardial depression |
|
|
myocardial depression for respiratory vs. metabolic acidosis
|
-respiratory acidosis produces more rapid and profound myocardial dysfunction than metabolic, likely because in respiratory acidosis CO2 is increased, and because of CO2's ability to freely diffuse across cell membranes and exacerbate intracellular acidosis to a greater extent than metabolic acidosis
|
|
|
in what patients are the detrimental effects of acidosis accentuated?
|
-in patients with ischemic heart disease, or those with impaired sympathetic activity, such as those who are B-blocked or under general anesthesia
|
|
|
respiratory acidosis
|

-caused by a mismatch between carbon dioxide production and ventilatory elimination: occurs when drugs or diseases cause a decrease in alveolar ventilation causing an increase in PaCO2, leading to formation of carbonic acid and hydrogen ions, and a pH<7.35
|
|
|
how can patients with normal or even elevated total minute ventilation get respiratory acidosis?
|
-due to diseases lungs with markedly increased dead space, causing wasted ventilation due to aeration of lung regions that arent participating in gas exchange
|
|
|
drugs we use and respiratory acidosis
|
-volatile anesthetics, IV anesthetics, and opioids cause resp acidosis by blunting peripheral and central chemoreceptors response to CO2
|
|
|
what other patients can get respiratory acidosis?
|
-those with increased CO2 production or absorption, esp those who are mechanically ventilated or with limited ventilatory reserve
|
|
|
hyoventilation as the cause of respiratory acidosis
|
can be due to:
-upper or lower airway obstruction (asthma, COPD, sleep apnea, and tumors) -CNS depression (anesthetics, opioids, neurologic injury) -lung or chest wall restriction (scoliosis, obesity, extreme ascites, pneumothorax, pleural effusion) -decreased skeletal muscle strength (residual muscle blockers, myopathy, neuropathy, spinal cord injury, botulinum toxin) -intrinsic pulmonary disease (pneumonia, pulmonary edema, fibrosis, sarcoidosis) |
|
|
increased carbon dioxide production/reabsorption
|
due to:
-increased metabolic production of CO2 (MH, hyperthyroidism, hyperalimentation with high carb content) -rebreathing exhaled gases (exhausted soda lime, incompetent one-way valve in the anesthetic breathing system) -CO2 absorption from pneumoperitoneum (laparoscopic surgery) |
|
|
compensation for respiratory acidosis
|
-over hours to days to kidneys compensate by increasing the secretion of H+ ions and reabsorption and production of bicarb ions
-after a few days, the pH will be near normal, even with the inc PaCO2 |
|
|
so what does a nearly normal pHa despite an increased PaCO2 (>40 mmHg) mean?
|
confirms respiratory acidosis is chronic rather than acute
|
|
|
how much will pH change based on how much rise in CO2?
|
pH will decrease 0.08 for every acute 10 mmHg increase in PaCO2
for chronic, pH will return toward normal if the elevated PaCO2 is sustained |
|
|
bicarbonate change for changes in CO2 (acute and chronic)
|
acute: bicarbonate will increase 1 mEq/L for every acute 10 mmHg increase in PaCO2
chronic: bicarbonate will increase 4 mEq/L for every chronic 10mmHg increase in PaCO2 |
|
|
treatment of respiratory acidosis
|
-when the pHa is <7.2, need to intubate and mechanically ventilate
-need to be careful not to overventilate, especially in patients who have chronic respiratory acidosis (because they already have compensation and the pH may be near normal, so hyperventilating will cause an alkalosis) |
|
|
what can metabolic alkalosis due to overventilation lead to?
|
-can lead to CNS irritability, and the vasoconstriction due to hypocapnea can cause CNS and myocardial ischemia
|
|
|
treatment of metabolic alkalosis associated with chronic respiratory acidosis?
|
-is with avoidance of overventilation, and with IV potassium chloride or acetazolamide
|
|
|
respiratory alkalosis
|
-when there is increased alveolar ventilation in relation to CO2 production, causing a low PaCO2 relative to bicarb levels, causing a pHa>7.45
|
|
|
causes of respiratory alkalosis
|
can be caused by:
-increased elimination of CO2 -decreased production of CO2 |
|
|
things that cause increased production of CO2
|
-iatrogenic (mechanical ventilation)
-drugs (doxapram, salicylates) -pregnancy -pain or anxiety -decreased barometric pressure -CNS injury -arterial hypoxemia -pulmonary vascular disease (PE) -liver cirrhosis -sepsis -hyperthermia-induced hyperventilation |
|
|
causes of decreased CO2 production
|
-hypothermia
-hypothyroidism -skeletal muscle paralysis by a muscle blocker |
|
|
metabolic comensation for respiratory alkalosis
|
^pHa = 0.008 x ^PaCO2 (acute)
^pHa = 0.017 x ^PaCO2 (chronic) |
|
|
consequences of respiratory alkalosis under normal circumstances
|
-the low CO2 and increased pHa decreases the stimulus to breath, as mediated by peripheral and central chemoreceptors
|
|
|
changes seen with chronic respiratory alkalosis
|
-bicarb ions are actively transported out of the CSF, causing the central chemoreceptors to reset to a lower PaCO2
-this same mechanism occurs during mechanical ventilation of the lungs during general anesthesia, causing spontaneous ventilation to be initiated at a lower PaCO2 -this also occurs when there is continued hyperventilation seen when returning to sea level from altitude, reflecting the central chemoreceptors being exposed to low bicarb CSF |
|
|
compensation for respiratory alkalosis
|
-by decreasing reabsorption of bicarbonate from the renal tubules, causing increased bicarb excretion in the urine, retuning the pH back toward normal
-alkalosis also stimulates the action of phosphofructokinase, which causes glycolysis and generation of lactic acid |
|
|
why is there tetany associated with respiratory alkalosis?
|
due to hypocalcemia secondary to the greater affinity of plasma proteins for calcium ions in an alkaline pHa
|
|
|
treatment of chronic respiratory alkalosis
|
-directed at correcting the underlying disorder
-during anesthesia, can easily decrease total minute ventilation |
|
|
metabolic acidosis
|
-when accumulation of any acid other than CO2 causes pHa <7.35
|
|
|
what starts immediately after development of metabolic acidosis?
|
-minutes after an increase in minute ventilation to eliminate CO2 starts, though keep in mind some patients may be physically unable to evoke this response
|
|
|
how can you clarify if the acidosis is primary or compensatory in response to respiratory alkalosis?
|
measuring the PaCO2
|
|
|
categories of metabolic acidosis for diagnostic purposes
|
into increased anion gap, and normal anion gap
|
|
|
anion gap
|
-the measured concentration difference between sodium cations and the sum of chloride and bicarbonate anions
-it represents the concentration of anions, both known and unknown, in the serum which are unaccounted for in the equation |
|
|
normal anion gap
what normally contributed primarily to the anion gap? |
3-11 mEq/L
-normally primarily contributed to by anionic serum albumin |
|
|
patients with low serum albumin
|
expected to have lower anion gaps
|
|
|
what does metabolic acidosis with an elevated anion gap suggest?
|
(>11, or less in hypoalbuminemic patients)
-suggests the accumulation of unmeasured anions (lactic acid or ketoacids) |
|
|
metabolic acidosis with a normal anion gap
|
-suggests bicarbonate wasting by the kidneys (renal tubular acidosis) or GI tract (diarrhea)
|
|
|
causes of increased anion gap metabolic acidosis
|
-drugs (methanol, ethanol, salicylates)
-ketoacidosis (alcohol, DM, starvation) -lactic acidosis (anaerobic glycolysis due to hypoperfusion of tissue during hypovolemia, heart failure, or cardiac arrest; cyanide toxicity; sepsis) -renal failure (accumulation of organic acids) -liver failure/cirrhosis (decreased conversion of lactate to glucose) |
|
|
causes of normal anion gap metabolic acidosis
|
-overzealous admin of 0.9% normal saline, or hyperalimentation
-GI bicarb losses (diarrhea, ileostomy, pancreatic fistula, neobladder) -renal bicar losses (renal tubular acidosis) -drugs (acetazolamide) |
|
|
ventilatory compensation for metabolic acidosis
|
expected PaCO2 = 1.5 x HCO3- + 8 (+/-2)
|
|
|
how does overzealous admin of 0.9% normal slaine caused normal anion gap metabolic acidosis?
|
by excessive chloride administration leads to imparement of bicarb reabsorption in the kidneys
|
|
|
compensatory response to metabolic acidosis
|
-includes increased alveolar ventilation because of stimulation of the carotid body chemoreceptors by H+ ions, and renal tubular secretion of H+ ions in the urine
-keep in mind that as with respiratory acidosis, anesthetics block the response of the chemoreceptors |
|
|
what is another mechanism of compensation in metabolic acidosis, and what are the consequences of this?
|
-the use of buffers in the bone to neutralize the nonvolatile acids
-the consequence is that in chronic metabolic acidosis, as in chronic renal failure, is often associated with loss of bone mass |
|
|
treatment of metabolic acidosis
|
-guided at diagnosis and treatment of the underlying cause
-can increase minute ventilation in a mechanically ventilated patient to temporarily compensate until treatment can take effect -can give bicarb, though its controversial |
|
|
bicarbonate administration
|
-can be used as a temporizing measure if pH <7.1 and the patient is deteriorating
-since giving bicarb generates CO2, should only give it to mechanically ventilated patients, because if it isnt eleimated by ventilation it can worsen intra and extra cellular acidosis -therefore it should NEVER be given in the setting of respiratory acidosis |
|
|
approach to giving bicarb
|
-it should be given on a trial basis, slowly give half the calculated dose and repeat the pHa and monitor hemodynamics to determine its impact
|
|
|
sodium bicarb dose calculation
|
-is based on deviation of the biacrb from normal
sodium bicarb dose= body wt (kg) x deviation of plasma bicarb from 24 x extracellular fluid volume as a fraction of body mass (0.3) EX: a 70kg pt with plasma bicarb of 12 mEq/L would require (70)(24-12)(0.3)= 252mEq sodium bicarb. Half is 126 mEq |
|
|
preparing an infusion of sodium bicarb
|
-dilute 3 ampules in a 1L bag of 5% dextrose in water
-this gives a sodium [] of 150 mEq/L, similar to that of 0.9% normal saline (154 mEq/L) -1 ampule of sodium bicarb has 50 mEq of sodium |
|
|
newer agents
|
-there are newer alkalinizing agents which dont generate CO2, but they have not been shown to decrease mortality in the setting of severe metabolic acidosis
include: -carbicarb, equimolar sodium carbonate and sodium bicarbonate -THAM |
|
|
what is the problem with alkalinizing agents?
|
because of their osmotic properties, they run the risk of causing hypervolemia and hypertonicity
|
|
|
adverse effects of acidosis, both respiratory and metabolic
|
-hyperkalemia
-CNS depression -cardiac dysrhythmias -hypovolemia (due to decreased precapillary and increased postcapillary sphincter tone) |
|
|
metabolic alkalosis
|
-when pHa > 7.45
-due to a gain in bicarbonate ions or loss of hydrogen ions |
|
|
causes of metabolic alkalosis
|
-conversion of citrate to lactate by the liver after large volumes of whole stored blood
-hypovolemia (think of this in post-op pts who develop metabolic alkalosis) -it also correlates to decreased chloride and potassium ions, as seen with diuretic therapy -excessive loss of H+ ions, during vomiting or GI suction -excessive production of bicarb ions: metabolism of LR, citrate in stored blood, acetate in hyperalimentation solutions) -hyperaldosteronism -permissive hypercapnea, as in underventilation in the long term mechanically ventilated patients with ARDS |
|
|
ventilatory compensation for metabolic alkalosis
|
expected PaCO2= 0.7 x HCO3- + 20 (+/- 1.5)
|
|
|
compensation for metabolic alkalosis
|
incldues:
-increased reabsorption of hydrogen ions by renal tubular cells -decreased secretion of hydrogen ions by renal tubules -hypoventilation |
|
|
what does renal compensation for metabolic alkalosis depend on?
|
-it depends on the presence of cations, Na and K, and chloride
-when these ions are depleted, as with vomiting, the kidneys ability to excrete excess bicarb is impaired, resulting in incomplete renal compensation for metabolic alkalosis |
|
|
hypoventilation as compensation for metabolic alkalosis
|
-the increase in CO2 will initially stimulate medullary chemoreceptors, offsetting the compensatory effect of decreased ventilation; in time, however, the CSF pH will normalize by active transport of bicarb ions into the CSF, and the volume of ventilation will decrease despite the increasing CO2
-if the PaCO2 rises again, however, the CSF pH will decrease and the sequence will be repeated |
|
|
notes about respiratory compensation for metabolic alkalosis
|
-in contrast to metabolic acidosis, respiratory compensation for pure metabolic alkalosis is never more than 75% complete, which means the pHa stays increased in patients with primary metabolic alkalosis
|
|
|
what then does a PaCO2 > 55 mmHg mean?
|
-this value is beyond the normal compensatory mechanism for metabolic alkalosis, and reflects concominant respiratory acidosis
|
|
|
treatment of metabolic alkalosis
|
-resolve the thing responsible, and in most cases giving saline with KCL, which allows the kidneys to excrete excess bicarb (as in chloride-sensitive alkalosis)
-occasionally, in chloride-resistant alaklosis) IV administration of H+ ions as ammonium chloride or hydrochloric acid, or the diuretic acetazolamide can facilitate return of pHa to normal |
|
|
administration of acid
|
-requires a central line, since doing it through a peripheral IV causes vein sclerosis and hemolysis
|
|
|
life-threatening metabolic alkalosis
|
rarely seen
|
|
|
ABG sampling
|
-most often done percutaneously from the radial, brachial, or femoral artery
|
|
|
peripheral venous blood as an alternative to arterial blood for ABGs
|
-venous blood from the dorsum of the hand becomes somewhat arterialized under the vasodilating effects of general anesthesia, so it can be used for the PCO2, pH, and base excess measurements
-it CANNOT, however, be used to estminate PaO2, since venous blood has variable lower PO2 than PaO2 |
|
|
what can venous PO2 be used to do though?
|
if it is high enough, it can be used to rule out hypoxemia (venous PO2 >60 mmHg)
|
|
|
venous PCO2 and pH compared to arterial
|
-PCO2 is 4-6 mmHg higher
-pH is 0.03-0.04 lower |
|
|
drawing blood for an ABG
|
-blood is drawn into a glass or plastic syringe with enough heparin to fill the dead space
-air bubbles have to be eliminated because equilibration of oxygen and CO2 in the blood with the corresponding air bubbles could influence the measured results |
|
|
heparin in the vial
|
-heparin is acidic, and excessive amounts could falsely lower the pH
|
|
|
why is the sample put on ice?
|
-unlike with RBCs, WBCs in the ABG sample are metabolically active, and use O2 and make CO2, so can cause a small error
-if the WBC count is elevated, such as in leukemia, a large decrease in O2 can occur, causing the sample to appear hypoxemic even though there is adequate oxygenation (leukocyte larcency) |
|
|
temperature correction of ABGs
|
-cooling blood makes it more alkaline due to increased CO2 solubility, which causes PaCO2 to decrease, and causes decreased H2O dissociation into H+ and OH-
-there is an issue to how best to manage pHa in patients under extreme hypothermia for EX: a pt cooled to 25C under cardiopulmonary bypass if left untreated will have a pH of 7.6, but warming the blood sample to 37C, which the ABG machines do, will cause a decrease in the measured pHa to 7.4 -there are 2 schools of thought about temp correction of ABG valuve, the alpha stat and pH stat |
|
|
alpha stat
|
-alpha refers to the protonation state of the a-imidazole side chain of histidine; the pKa of histidine changes with temperature so that its protonation state is relatively constant regardless of temp, and thus by allowing the patient's pHa to drift with temperature you are allowing the protonation state of the histidine resiudes to remain static (hence "stat")
-this arose from the observation of cold blooded animals, which rely on a similar complement of enzymes as warm blooded, function well over a wide range of body temperatures |
|
|
pH stat
|
-more involved technique than alpha stat, which maintains the patients pH at 7.4, by modifying PaCO2, regardless of temperature
-the lower pHa maintained may result in improved cerebrovascular perfusion during hypothermia, though there is a debate over which leads to better outcomes (alpha or pH) |
|
|
PaO2 and temperature changes
|
-it is routine for blood gas analyzers to do so at a temp of 37C, regardless of the patients body temp
the only thing that is important is to temperature correct the PaO2: -PaO2 should be decreased 6% for every 1C the patients temp is below 37C -PaO2 should be increased 6% for every 1C the temp is above 37C -the A-a gradient also requires correction |
|
|
alveolar gas equation and alveolar-to-arterial oxygen gradient calculation
|
alveolar gas equation:
PAO2= (PB - PH2O) - PaCO2/RQ alveolar-to-arterial oxygen gradient: A-aDO2= PAO2 - PaO2 A-aDO2= alveolar-to-arterial O2 gradient (mmHg) PAO2= alveolar partial pressure oxygen (mmHg) PaO2=measured arterial partial pressure O2 PB= barometric pressure (760 mmHg at sea level) PH2O=partial pressure of water vapor (47 mmHg at 37C) FIO2= inspired oxygen concentration PaCO2= arterial partial pressure of CO2 RQ: respiratory quotient (relative CO2 produced per mL oxygen consumed) = 0.8 |
|
|
example of the alveolar gas equation and A-a gradient equation
|
Arterial blood gas analysis reveals a PaO2 of 310 mmHg and a PaCO2 of 40 mmHg while breathing 100% oxygen (FIO2 = 1.0). PB is 760 mmHg, and PH2O is 47 mmHg
PAO2= (760-47)1.0 - 40/0.8 PAO2= 713 - 50 PAO2= 663 mmHg A-a DO2= 663 - 310 A-a DO2= 353 mmHg (normal <60 mmHg) Each 20mmHg of A-aDO2 represents venous admixture equivalent to 1% of cardiac output; 353/20 = 18% of cardiac output shunted past the lungs |
|
|
oxygen measuring electrode for ABGs
|
-the O2 electrode, or Clark electrode, is a polarographic cell consisting of a silver reference anode and a platinum cathode charged to 0.5 V
-the platinum surface is covered with an oxygen-permeable membrane (polyethylene), on the other side of which is placed the unknown sample -the electric current passing thropugh the polarographic cell is directly proportional to the PO2 outside the membrane |
|
|
carbon dioxide electrode
|
-the Severinghaus electrode, has a CO2 permeable membrane (Teflon) which permits CO2 to diffuse from the unknown sample into a buffer solution containing bicarb ions bathing a conventional glass pH electrode, and the measured pH in the bathing solution is altered in direct proportion to PCO2
|
|
|
pH electrode
|
-a glass electrode that senses the concentration of H+ ions, which produce a proportional chang ein voltage between the glass and reference electrode
|
|
|
information an ABG provides
|
values to assess oxygenation and ventilation:
-pHa -PaO2 -PaCO2 additional measures that help further define oxygenation and ventilation: -A-a DO2 -arterial-to-alveolar PO2 ratio (a/A) -mxed venous PO2 -arterial and mixed venous content of oxygen -position of the oxyhemoglobin dissociation curve -the dead space-to-tidal volume ratio (VD/VT) |
|
|
how is oxygenation assessed?
|
by measuring the PaO2
|
|
|
arterial hypoxemia
|
PaO2< 60 mmHg
|
|
|
causes of hypoxemia
|
-a low PO2 in inhaled gas (altitude, accidental occurance during anesthesia)
-hypoventilation -venous admixture (with or without decreased mixed venous oxygen content) |
|
|
O2 at altitude
|
-atmospheric oxygen is a constant 21% of barometric pressure, but barometric pressure dominishes with altitude, so the partial pressure of oxygen also diminished
-furthermore, the water vapor pressure generated by the humidification of gases in the lungs is greater fraction of partial pressure at altitude, making "less room" for oxygen -the alveolar gas equation accounts for both of these variables: PAO2= (PB - PH2O)FIO2 - PaCO2/RQ |
|
|
decreased PaO2 due to hypoventilation
|
-is due to the rising CO2 concentration in the alveoli, which dilutes the oxygen concentration as the CO2 encroaches on the space available for oxygen in the alveolus
-the alveolar gas equation estimates the decrease in alveolar oxygen, and decreases in PaO2 are roughly equivalent to increases in alveolar PCO2 (PACO2) |
|
|
venous admixture
|
--the mixture of deoxygenated venous blood with oxygenated arterial blood
-due to some sort of right-to-left shunt which can be due to the lungs (atelectasis, pneumonia, endobronchial intubation) or the heart (VSD, etc) -normally there is a small fraction of venous blood always shunted, but <1%; with significant shunting (50%) inhalation of 100% O2 produces minimal effect on PaO2 -the effects of this will be magnified in anemic blood (with decreased oxygen carrying capacity) or increased oxygen consumption during low cardiac output states |
|
|
how can the magnitude of venous admixture or shunting be estimated?
|
by calculating A-aDO2
-this estimates the difference in oxygen partial pressure between the alveoli (PAO2) and arterial blood (PaO2) -there is always a small difference of 5-10 mmHg due to shunting via the thebesian and other veins -greater differences suggest presence of pathologic shunting, either intra or extrapulmonary 0keep in mind it increases with age, with elevated inspired oxygen concentration (up to 60 mmHg when breathing 100% O2), and with vasodilating drugs (nitroglycerine, nitroprusside), which impair the hypoxic pulmonary vasoconstriction |
|
|
using pulse ox in these situations
|
-provides false reassurance in pts breathing high inspired oxygen concentrations, for example a pulmnary process causing significant shunting can exist despite an oxygen saturation of 100%
-in this situation its best to calculate the A-aDO2 |
|
|
what does the A-aDO2 roughly estimate?
|
the shunt fraction of cardiac output
|
|
|
A-aDO2 and the percent of CO being shunted
|
-for PaO2 higher than 150 mmHg, such that Hb is completely saturated with O2, the magnitude of venous admixture is about 1% of CO for every 20 mmHg of A-aDO2
(below 150 mmHg or when CO is increased relative to metabolism, this guideline will underestimate the actual amount of venous admixture) |
|
|
what is the disadvantage of the A-aDO2?
|
the normal range changes with varying concentrations of inspired oxygen
|
|
|
how can this problem be solved?
|
use the a/A ratio, which remains relatively constant regardless of inspired concentrations of oxygen
Ex: a patient with an a/A ratio 0.5 will have a PaO2 equal to 50% of the PAO2 |
|
|
calculating the a/A ratio
|
alveolar-to-arterial oxygen ratio:
a/A= PaO2/PAO2 Example: Arterial blood gas values are a PaO2 310 mmHg and a PaCO2 of 40 mmHg while breathing 100% oxygen (FIO2 = 1.0). PB is 760 mmHg and PH2O is 47 mmHg PAO2= (760-47)1.0 - 40/0.8 PAO2= 713-50= 663 mmHg a/A= 310/663 a/A= 0.47 (normal >0.75) |
|
|
what is a simple alternative to the a/A ratio?
|
the PaO2/FIO2 ratio
-it also remains constant over a range of inspired oxygen concentrations |
|
|
PaO2/FIO2 values
|
-hypoxemia is defined as a value <300
-<200 suggests a shunt fraction greater than 20% |
|
|
mixed venous blood sample
|
-obtained from the distal part of an unwedged pulmonary artery catheter
|
|
|
what is mixed venous blood PO2 a function of?
|
both cardiac output and tissue oxygen consumption
|
|
|
normal value of mixed venous PO2
|
40 mmHg
|
|
|
what can be said about mixed venous PO2 in the presence of unchanging tissue oxygen consumption?
|
it varies directly with CO: when there is a decreased CO there is less blood flow available for tissue oxygen extraction, therefore the tissues extracting the same amount of O2 will result in a decreased mixed venous PO2 because they have more time to extract the O2
|
|
|
what does a mixed venous PO2 < 30 mmHg suggest?
|
tissue hypoxemia
|
|
|
and a high mixed venous PO2?
|
diseases associated with arterial-venous admixture, like peripheral shunting (sepsis, portal hypertension) or impaired oxygen use (cyanide toxicity)
|
|
|
using arterial and mixed venous content of O2
|
-the vast majority of the O2 in the blood is bound to Hb, and the difference between the arterial and mixed venous O2 content is an estimate of the adequacy of CO relative to tissue oxygen consumption
-the normal difference is 4-6 mL/dL, but when tissue oxygen consumption is constant and CO drops, there will be a greater difference between the arterial and mixed venous O2 content because of the increased oxygen extraction |
|
|
how is CO estimated when assumptions are made about oxygen consumption?
|
using the Fick equation:
VO2 = CO x (CaO2 - CVO2) VO2=oxygen consumption (about 3.5 ml/kg/min) CO= cardiac output |
|
|
arterial and venous oxygen content equations
|
arterial oxygen content (ml/dl):
CaO2= (Hb x 1.39)Sat + PO2(0.003) venous oxygen content (ml/dl): CVO2 = (Hb x 1.39)Sat + PaO2 (0.003) -Hb= Hb (g/dl) -1.39=oxygen bound to Hb (ml/g) -Sat=% oxygen saturation of Hb -PaO2=arterial partial pressure of oxygen (mmHg) -PvO2= mixed venous partial pressure of oxygen (mmHg) -0.003=dissolved oxygen (ml/dl x mmHg) |
|
|
example of using these equations
|
Hb=15 g/dl, PaO2= 100mmHg (resulting in nearly 100% saturation), PVO2 = 40 mmHg (resulting in 75% saturation)
CaO2= (15x1.39)1.00 + 100(0.003) = 20.85 + 0.3= 21.15 ml/dl CVO2= (15x1.39)0.75 + 40(0.003) = 15.63 + 0.12 = 15.75 ml/dl CaO2 - CVO2 = 5.4 ml/dl oxygen consumption= 250 ml/min= COx5.4 ml/dl CO= 250/5.4= 46 dl/min = 4.6 L/min |
|
|
the oxyhemoglobin dissociation curve
|
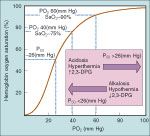
-describes the saturation of Hb with oxygen relative to PO2
|
|
|
what is the PO2 at a 50% saturation?
|
26 mmHg, referred to as the P50
|
|
|
things that shift the curve to the left (P50 lower than 26 mmHg)
|
-things which may jeopardize tissue oxygenation because oxygen is more tightly bound to Hb and so PaO2 must decrease more than normal to have O2 release from Hb
include: -alkalosis -hypothermia -decreased 2,3 DPG |
|
|
shifting the curve to the right (P50 >26 mmHg)
|
-facilitate tissue oxygen availability by permitting the unloading of oxygen from Hb at an increased PaO2
include: -acidosis -hyperthermia -increasing 2,3 DPG |
|
|
normal PO2 at 75% sat
|
40 mmHg
|
|
|
PO2 at 90% sat?
|
60 mmHg
|
|
|
when is saturation 100?
|
when the PO2 is greater than 150 mmHg
|
|
|
the body's responses to hypoxia
|

-acute hypoxia causes activation of the sympathetic nervous system with endogenous catecholamine release, which augments BP and CO, despite the vasodilating effects of hyoxemia; the increased CO increases O2 delivery from the lungs to the tissues, and if tissue oxygen consumption remains unchanged, the oxygen content of the returned venous blood will be higher
-also, a less efficient compensation will be an increase in alveolar oxygen concentration and PaO2 by lowering the alveolar and arterial carbon dioxide concentration (though the increased oxygen consumption by the respiratory muscles may offset the gain -these responses are initiate by the chemoreceptors in the carotid bodies, and will be blunted by volatile anesthetics |
|
|
what does PaCO2 reflect?
|
the adequacy of ventilation to remove CO2 from the pulmonary capillary blood
|
|
|
what is PaCO2 directly proportional to in the steady state?
|
-to the metabolic production of CO2, whereas its inversely proportional to alveolar ventilation
|
|
|
what does the production of CO2 depend on, and what does it parallel?
|
depends on the metabolic state of the person, and parallels the consumption of oxygen
|
|
|
under normal conditions, how much CO2 is produced for oxygen consumed?
|
80% as much CO2 is produced as O2 consumed (hence the respiratory quotient is 0.8)
|
|
|
what does the VD/VT reflect?
|
-the fraction of each tidal volume that aerates regions of the lung or respiratory tract not involved in gas exchange (for example increased dead space markedly decreases the efficiency of ventilation)
|
|
|
normal VD/VT value in the upright position
|
should not exceed 0.3
|
|
|
what can completely overcome increased dead space?
|
increased alveolar ventilation (unlike problems with oxygenation, which this wont overcome)
|
|
|
impact of venous admixture on PaCO2
|
-unlike PaO2, it has no impact because of the extreme diffusibility of CO2 relative to that of oxygen
|

