![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
119 Cards in this Set
- Front
- Back
|
conversion between eV and cm-1 |
1eV = 8066cm-1 |
|
|
gross structure |
electron and nuclear attraction electron electron repulsion electron kinetic energy |
|
|
fine structure |
spin orbit interactions relativistic corrections |
|
|
hyperfine structure |
nuclear attractions |
|
|
wavenumber |
1/wavelength |
|
|
reduced mass |
1/m = 1/me + 1/mn |
|
|
energy using rydbergs constant |
En = - Rh/n^2 = |
|
|
R infinity hc |

|
|
|
equations for the transition between states |

|
|
|
equations for bohr radius and fine structure constant |

|
|
|
angular momentum operator |

|
|
|
probability in terms of radial function |
P(r) =r^2R(r)^2 |
|
|
eigenfunction of L^2 operator |

|
|
|
values of ml |

|
|
|
normalisation |
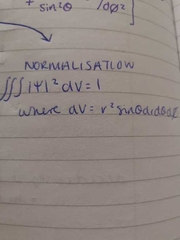
|
|
|
electric dipole |
2 oppositely charged charges separated by a distance vibrations of electrons. create fluctuating dipole |
|
|
spontaneous emissions |
emission of a photon when an atom de-excites |
|
|
homogenous broadening |
affects all indivual atoms in the same way natural and collision broadening |
|
|
inhomogeneous broadening |
affects atoms in different ways found in solids where atoms experience different local environments |
|
|
assumption for atoms |
that they are spherical symmetric |
|
|
equation for electric dipole |
p=qd |
|
|
fermi's golden rule |
W12 = (2pi/hbar)|M12|^2 g(hf) |
|
|
equation for parity |
parity = (-1)^L |
|
|
Einstein constant A |
A=1/tau |
|
|
equation for the time between collisions |

|
|
|
observable frequency for doppler broadening |
f = f0(1±vx/c) |
|
|
non radiative transition time |
1/tau = A + 1/tau(non radiative) |
|
|
polarised light in terms.of perturbation |

|
|
|
collision broadening |
gas collides with the wall and interrupts the emission of light uncertainty principle |
|
|
selection rules |

|
|
|
natural broadening |
photon emitted causes a burst of light that decays exponentially caused lorentzian line delta E delta t > h bar |
|
|
doppler broadening |
thermal motion doppler shift in frequency |
|
|
aufbau principle |
the filling up of shells in order of increasing every |
|
|
quantum defect |
allows for perturbation of inner shells by the valence electrons |
|
|
bohr formula |

z can be z effective |
|
|
types of series |
k series n=1e has been ejected l series n=2e m series n=3e |
|
|
shell model |

|
|
|
evidence for Shell model |

|
|
|
absorption edge |

|
|
|
equation for quantum defect |

|
|
|
screening |

|
|
|
emission spectra |

|
|
|
spin orbit coupling |

|
|
|
force on a magnetic dipole |
Fz = uz (dB/dz) |
|
|
spin momentum |

|
|
|
spin orbit interaction and types of coupling based on interaction |

|
|
|
what happens to L+S when there are 2 electrons |
has to be even |
|
|
evidence for spin |

|
|
|
LS coupling |

|
|
|
shorthand |

|
|
|
rules for ground state (hunds rules) |

|
|
|
helium wavefunction |

|
|
|
Pauli exclusion principle |
absense of a triplet is the same as trying to put 2e- in same state |
|
|
exchange energy |
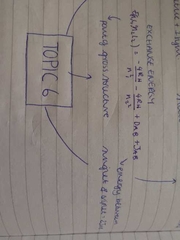
|
|
|
gyrometric ratio (gamma) |
e/2m it specifies the proportionally constant between angular momentum and its magnetic moment |
|
|
Thomas precession |
reduces energy by a factor of 2 |
|
|
interval rule |
levels with same L and S but different J are separated by an energy that is proportional to J |
|
|
mass effects |
changes in nuclear mass make changes to reduced mass hence energies |
|
|
field effects |
electrons in s shell have a finite probability of penetrating the nucleus therefore sensitive to charge distribution |
|
|
comparison of nucleus to electrons |

|
|
|
total momentum including nuclear momentum |

|
|
|
magnetic moment and derivation |
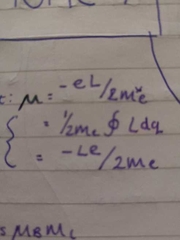
|
|
|
magnetic moment z component |

|
|
|
magnetic field in z |
Bz = u0I/2r |
|
|
Central field |

|
|
|
spin on nucleus |
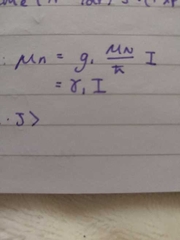
|
|
|
hyperfine energy |
<I. J> |
|
|
normal zeeman effect |

|
|
|
longitudinal observation of normal. zeeman effect |

|
|
|
transverse observation of normal zeeman effect |

|
|
|
quantum confined stark effect |
exciton from optical absorption binding energy is small very unstable |
|
|
pashen back effect |

|
|
|
anomalous zeeman effect |

|
|
|
quadratic stark effect |
small redshift is proportional to root energy |
|
|
linear stark effect |

|
|
|
definition of laser |
an oscillator as well as an amplifier amplification achieved by stimulated emission |
|
|
losses from a laser |

|
|
|
positive feedback diagram |
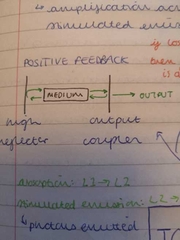
|
|
|
condition for oscillation |

|
|
|
A and B coefficients of emission and absorption |

|
|
|
population inversion |

|
|
|
four level system |

|
|
|
three level system |
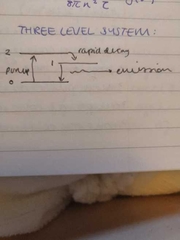
|
|
|
define cavity |
provided positive feedback that turns an amplifier into an oscillator |
|
|
define transverse modes |
describe the variation if the E field across a cross section of a beam. |
|
|
define longitudinal modes |
determines emission spectra |
|
|
multimode operation |
for a given longitudinal mode to oscillate, its frequency must lie within the emission spectra |
|
|
single mode operation |
make laser with one mode |
|
|
mode locking |
emission of a continuous train of short pulses |
|
|
active technique |
time independent shutter opened every 2L/c seconds |
|
|
passive technique |
saturable absorber put into cavity |
|
|
spatial coherence |
related to phase uniformity across a cross section high in single modes |
|
|
temporal coherence |
refers to time duration over which the phase is constant |
|
|
H n and m values |

|
|
|
Length of cavity |
L = int x c/2nf |
|
|
frequency in cavity |
f = int x c/2nl |
|
|
minimum pulse duration |
delta t min delta f > 1/2pi |
|
|
temporal coherence time |
tc = 1/detla f |
|
|
coherence length |
lc = ct = c/delta f |
|
|
root mean squared velocity |
v = root(3kt/m) |
|
|
doppler shifted frequency |

|
|
|
max force on an atom |
Fx = - h/lamda tau |
|
|
deceleration |
ax = - h/mlamda tau |
|
|
cycles to stop an atom |
N =mu(x) lamda/h |
|
|
min time to stop an atom |
tmin = N(stop) x tau |
|
|
min distance travelled to stop an atom |
d = u(x) ^2/2a^2 |
|
|
doppler limit temperature |
T = h bar /2Kb tau |
|
|
recoil temperature |
T = h^2/mKlamda^2 |
|
|
debroglie wavelength |
lamda = h/root(3mkT) |
|
|
wavefunction overlap |
N/V = 1/debroglie wavelength ^2 |
|
|
temperature of condensation |
Tc = 1/3 h^2/mk (N/V) ^2/3 |
|
|
BEC |

|
|
|
optical molasses |
fix laser beam arrangement to. stop atoms moving in all 3 directions |
|
|
magneto-optical trap |
add magnetic coils above and below laser beams |
|
|
low field seeking |
experience potential minimum at the centre traps then close to origin |
|
|
sisphysis cooling |
atoms repeatedly climb to top. of. potential created by stark effect and then drop down after absorption and emission of a photon |
|
|
procedure of BEC |

|
|
|
what does doppler cooling ignore |
stimulated emission |
|
|
temp comparison between optical molasses and doppler limit |
optical molasses cools atoms to below temp of doppler limit. |

