![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
What does increasing the pressure do to the rate of reaction? |
It increases the concentration which means there are physically more particles in a certain area. This increases the frequency of collisions therefore increasing the rate of reaction. |
|
|
Describe the bonding of a simple covalent substance. |
Strong covalent bonds between the atoms, weak intermolecular forces giving a low boiling point. |
|
|
Describe the bonding in an ionic compound. |
Strong bonds between each ion in a giant irregular lattice giving a high melting point. |
|
|
What shapes are the best for catalysts and why? |
Powders as they particles have a larger surface area to react with. |
|
|
In the electrolysis of aluminium oxide, why does the anode have to be replaced? |
The oxygen reacts with the anode to produce carbon dioxide. |
|
|
In what state will ionic compounds conduct electricity and why? |
In solution or molten and the ions can move and carry a charge. |
|
|
Why are nanoparticles good catalysts? |
They have large surface area to volume ratios and react quickly. |
|
|
How do you work out an empirical formula? |
Experimental mass ÷ relative atomic mass for each substance then find the ratios. |
|
|
What are the properties of a thermosoftening polymers? |
Tangled chains of polymers, no crosslinks, weak intermolecular forces. Therefore a low melting point. |
|
|
Why is aluminium dissolved in molten cryolite for electrolysis? |
It has a lower melting point which saves energy and money. |
|
|
Why are alloys harder than pure metal? |
They have an irregular lattice so the atoms cannot slide over each other easily. |
|
|
Acid + Alkali =? |
Salt and Water |
|
|
Why are metals such good conductors? |
They have delocalised electrons that can carry a charge anywhere in the lattice. |
|
|
Describe the bonding between group 1 elements and group 7 elements. |
Group elements lost 1 electron and become positively charged and the group 7 elements gain an electron become negatively charged. The two are strongly attracted to each other and bond together. |
|
|
Why are giant covalent substances used in areas with high working temperatures? |
They have lots of strong covalent bonds which require lots of energy to breaks giving them high melting points. |
|
|
What is chlorine used for? |
Cleaning swimming pools and drinking water. |
|
|
What is the chemical equation for a neutralisation reaction? |
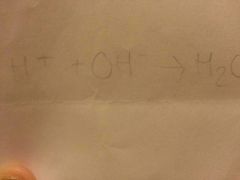
|
|
|
In electrolysis, will the most or least reactive ion come off the cathode first? |
The least reactive |
|
|
What ion causes a ph less than 7? |
H+ |
|
|
What ion causes pH greater than 7? |
OH- |
|
|
Why do substances seperate in gas chromatography? |
Particles move a different speeds. |
|
|
What questions cannot be answered by science alone? |
Social Ethical Economic |
|
|
What does HCL stand for and what salt does it make? |
Hydrochloric acid. It makes chlorides. |
|
|
Why would you not react potassium with an acid? |
Potassium is extremely reactive and would be too dangerous. |
|
|
What salt does nitric acid produce? |
Nitrates |
|
|
What salt does sulphuric acid produce? |
Sulphates |
|
|
Metal + Acid =? |
Salt + Hydrogen |
|
|
What happens to positive ions at the negative electrode? |
They gain electrons and are reduced to atoms. |
|
|
What does exothermic mean? |
Releases heat(energy) |
|
|
What does endothermic mean? |
Takes in/requires energy |
|
|
Why does graphite conduct electricity? |
It has delocalised electrons. |
|
|
Why is graphite so weak? |
It has weak forces of attraction between the layers. |
|
|
How are ions used to work out the formula of a compound? |

|
|
|
What do covalent bond diagrams look like? |
|
|
|
What is the structure of a metal? |
Regularly arranged ions surrounded by a sea of delocalised electrons. |
|
|
What is activation energy? |
The amount of energy required to start a reaction. |
|
|
What are three ways of measuring rate? |
How quickly reactants are used up How quickly products are produced How quickly a change in mass occurs |
|
|
How do catalysts increase the rate of reaction? |
The reduce the required activation energy. |
|
|
Describe the structure and bonding of a diamond. |
Each carbon atom forms four strong covalent bonds in a giant irregular lattice forming a very strong structure. |
|
|
How do you work out relative formula masses? |
Add the atomic masses. |
|
|
What does a molecular ion peak tell you? |
The relative atomic mass of a substance |
|
|
What are the rules for electolysis in solution? |
Positive Electrode: Halides come off first. Then OH- ions as oxygen. Then any other negative ions. Negative Electrode: Least reactive positive ions first. If the ion is more reactive than hydrogen, hydrogen will come off first. |
|
|
How does gas chromatography work? |
Your chosen mixture is dissolved in a solvent and the injected into the machine. A non reactive carrier gas carries it through the machine. Since particles move at a different speed, the substances are separated and come out of the machine at different time where they are detected by a detector. |
|
|
What name is given to alloy with special properties? |
Smart alloys |
|
|
What are the four ways of increasing the rate of reaction? |
Surface area, temperature, concentration and catalyst. |
|
|
GC-MS is especially useful with what sized samples? |
Small |
|
|
What is a special type of electrolysis? |
Electroplating |
|
|
What is an element? |
A substance where all the atoms are the same. |
|
|
What is a compound? |
A substance where different atoms are chemically combined. |
|
|
What is ionic bonding between? |
A metal and a non-metal. |
|
|
Why are metals easy to bend? |
Their atoms can slide over each other easily. |
|
|
Why are instrumental methods good? |
They are fast, sensitive and there is less room for human error. |
|
|
Describe the process of fractional distillation. |
The mixture is boiled and vaporised. It the travels up the column and each substance is condensed and collected at a different temperature. |
|
|
What is a mixture? |
A blend of substances that aren't chemically combined. |
|
|
Why does increasing the temperature increase the rate of reaction? |
Increasing the temperature means the particles have more energy and therefore move faster. This increases the frequency of collisions therefore increasing the rate of reaction. |
|
|
What name is given to substances such as metal hydroxides that can neutralise acids? |
Bases |
|
|
What are soluble metal hydroxides called? |
Alkalis |
|
|
Base + Acid = ? |
Water + Salt |
|
|
What does increasing the surface area do to the rate of reaction? |
Increasing the surface area means that particles have a larger area to collide with. This increases the frequency of collisions and therefore the rate of reaction. |
|
|
Why do catalysts last for a long time? |
They are not used up in a reaction. |
|
|
How do you get salt crystals from a base and an acid? |
-Add excess base to acid -Filter the excess base -Boil the solution -Collect crystals |

