![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
199 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
The Urinary System |
The Urinary system is composed of the paired kidneys and the urinary tract. |
|
|
|
The kidneys |
The kidneys filter the blood to remove metabolic wastes and then modify the resulting fluid which allows organs to maintain fluid, electrolyte, acid-base and blood pressure homeostasis. |
|
|
|
The urinary tract |
organs that transport store and eventually elminate urine from the body. is composed of the paired ureters the urinary blader and the urethra. |
|
|
|
The kidneys |
situated against the posterior abdominal wall and are retroperitoneal organs, meaning they are located posterior to the peritoneal membranes.
|
|
|
|
position of the kidneys |
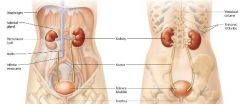
the left kidney extends from thoracic vertebrae 12 to lumbar vertebrae 3
the right kidney is slightly lower. |
|
|
|
Adrenal gland |
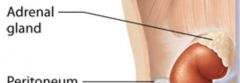
Each kidney is capped by an adrenal gland; these glands perform endocrine functions and secrete a variety of hormones. |
|
|
|
Ureters |
tubes that run along the posterior body wall, connecting the kidneys with the hollow urinary bladder. |
|
|
|
The bladder |
Stores urine and sits on the floor of the pelvic cavity. Urine is expelled from the body through the tube called the urethra, which connects the urinary bladder with the outside of the body. |
|
|
|
Regulation of urine by the kidneys |
Removal of metabolic wastes
Regulation of fluid and electrolyte balance
Regulation of acid base balance
Maintenance of blood pressure
Regulation of erythropoiesis
Performing other metabolic functions |
|
|
|
Removal of metabolic wastes |
As we have discussed, the kidneys filter the blood, removing metabolic wastes. These wastes are eliminated from the body via the urine. |
|
|
|
Regulation of fluid and electrolyte balance |
The kidneys regulate blood solute concentration or osmolarity by conserving or eliminating water and electrolytes such as sodium, potassium, and calcium ions. |
|
|
|
Regulation of Acid-Base balance |
The kidneys assist in the long term regulation of blood pH by conserving or eliminating hydrogen (H+) and bicarbonate (HCO3-) ions. |
|
|
|
Maintennce of Blood Presssure |
The kidneys directly influence systemic blood pressure through their control of blood volume. Additionally they secrete an enyme that influences both blood volume and peripheral resistance. |
|
|
|
Regulation of Erythropoiesis |
The kidneys regulate red blood cell production in the bon marrow by releasing the hormone erythropoietin. |
|
|
|
Performing other metabolic functions |
The kidneys play many important metabolic roles, including detoxifying susbtances in the blood, activating vitamin D,and making new glucose throgh the process gluconeogenesis. |
|
|
|
External connective tissue layers of the kidneys |
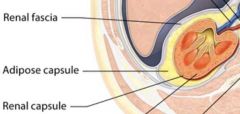
Renal Fascia
Adipose Capsule
Renal Capsule |
|
|
|
Renal Fascia |
The rena fascia is a layer of dense irregular connective tissue that anhors each kidney to the peritoneum and to the facia covering the muscles of the posterior abdominal wall. |
|
|
|
Adipose Capsule |
The middle and thickest layer, called the adipose capsule, consists of adipose tissue that wedges each kidney in place and shields it from physical shock. During prolonged starvation, the body used the fatty acids in the adipose capsule of the kidney for fuel. This causes the kdiney to droop, a condition called nephroptosis. |
|
|
|
Renal Capsule |
The renal capsule is an extermely thin layer of dense irregular connective tissue that covers the exterior of eah kidney like plastic wrap. It protects the kidney from infection and physical trauma. |
|
|
|
Size of a typical kidney |
a typical adult kidney is about the size of a large bar of soap, and weighs about 150 grams. |
|
|
|
The Hilum |
located on the medial surface, opening which the renal artery, renal vein, renal nerves and ureter enter and exit the kidney. The connective tissue anchors the ureter, blood vessels, and nerves in place. |
|
|
|
Renal Sinus |
central cavity which is lined by the renal capsule and filled with urine draining structures and adipose tissue. |
|
|
|
regions of the internal kidney |
The renal cortex
The middle renal medulla
Inner renal Pelvis |
|
|
|
The renal cortex and renal medulla |
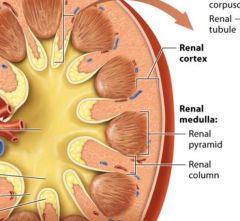
together the renal cortex and the renal medulla make up the urine forming portion of the kidney.
The renal cortex is reddish brown due to its rich blood supply it houses 90-95% of the kidneys blood vessels. The renal cortex and renal medulla of each kidney contain over on million microscopic filtering structures called nephrons. |
|
|
|
The renal pelvis |
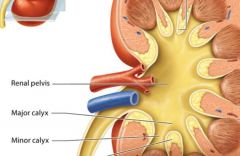
the renal pelvis and associated structures drain urine that the cortex and medulla have formed. |
|
|
|
Renal columns |
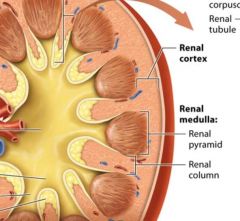
extensions of the renal cortex pass through the renal medulla toward the renal pelvis. The renal columns house blood vesels that branch from the renal artery as they travel to the outer portion of the cortex. |
|
|
|
Renal pyramids |
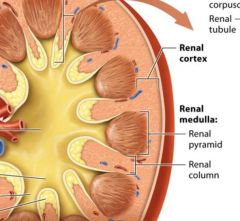
within the renal medulla cone shaped structures which are seperated from one another by a renal column on each side. The renal pyramids are darker in color and appear striped, reflecting that they are made up od parallel bundles of small tubes, with fewer blood vessels than in the renal cortex. |
|
|
|
Nephrons |
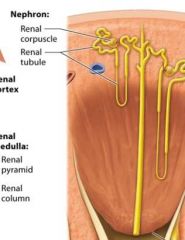
functional units of the kidney, each one is capable of filtering the blood and producing urine |
|
|
|
Main components of nephrons |
renal corpuscle renal tubule |
|
|
|
the renal corpuscle |
globe shaped stucture resides in the renal cortex. |
|
|
|
renal tubule |
long snaking tube of epithelium resides mostly in the renal cortex and also in the renal medulla |
|
|
|
Papilla |
located at the tip of each renal pyramid |
|
|
|
Minor calyx |
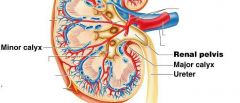
cup shaped tube, the first urine draining structure |
|
|
|
Major calyx |
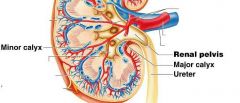
urine from three to four minor calyces drain into a larger major calyx.
Two to three major calyces in turn drain urine into the large collecting chamber that is the renal pelvis, which leads into the urter.
Smooth muscle tisue in the walls of the calyces and renal pelvis contracts to help propel urine toward the ureter. Both the calyces and the renal pelvis reside in the renal sinus. |
|
|
|
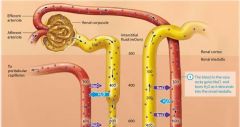
Blood supply to Kidneys |
Kidneys receive approximately one fourth of the total cardiac output about 1200 ml per minute from the right and left renal arteries which branch from the abdominal aorta. |
|
|
|
Renal arteries |
arteries that branch from the abdominal aorta and fan out into smaller vessels as they pass through the renal sinus to the renal columns and cortex. |
|
|
|
Arteris of the kidney from largest to smallest |
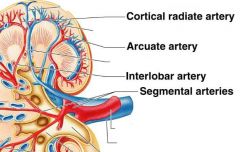
Renal artery
Segmental Artery
Interlobar Artery
Arcuate Artery
cortical radiate artery. |
|
|
|
Capillary beds of the kidneys |
in the kidneys we find an unusual capillary bed that is both fed and drained by arterioles. In the kidneys renal cortex, the interlobular arteris branch into tiny affernet arterioles. |
|
|
|
Afferent Arterioles |
feed a ball shaped capillary bed called the glomerulus. |
|
|
|
The glomerulus |
the glomerulus consists of glomerular capillaries and supporting cells and is part of the renal corpuscle of the nephron. |
|
|
|
Efferent Arteriole |
The glomerular capillaries are then drained by a second arteriole called the efferent arteriole. The efferent arteriole feeds a second capillary bed known as the peritubular capillaries. |
|
|
|
Peritubular capillaries |
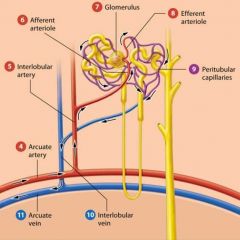
from a plexus (network) around the renal tubule of each nephron.
|
|
|
|
Order of blood flow |
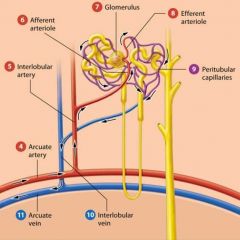
Affernet arteriole
Glomerulus
Efferent Arteriole
Peritubular Capillaries |
|
|
|
Venous blood flow from the kidney |
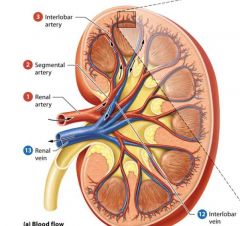
interlobular cortical radiate vein
Arcuate vein
Interlobar Vein Segmental vein
Renal Vein |
|
|
|
The renal vein |
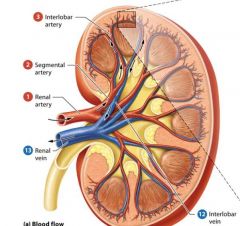
Exits the kidney through the hilum and empties into the inferior vena cava. |
|
|
|
The Nephron and Collecting System |
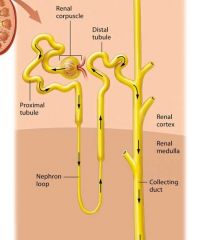
Most of the functions the kidneys occur in the nephrons.
Nephrons filter the blood and modify the filtered fluid as it passes through the renal tubules. This fluid then leaves the nephron and drains into the tubules of the collecting system, where it is further modified until it finally becomes urine.
|
|
|
|
The Renal Corpuscle |
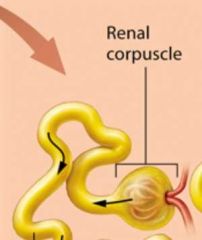
Responsible for filtering the blood. Each renal corpuscle consists of two parts: the glomerulus and an outer sheath of epitherlial tissue called the glomerular capsule. |
|
|
|
The Glomerulus |
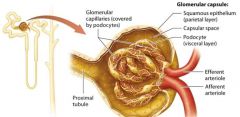
The glomerulus is a group of looping fenestrated capillaries. These capillaries are called fenestrated because of the large pores, or fenestrations, present within their plasma membranes and between their endolthelial cells. |
|
|
|
Function of Fenestrations |
fenestrations make the capillaries extermely leaky or permeable, and form a main part of the filtering structure of the renal corpuscle.
|
|
|
|
Glomerular Capsule |
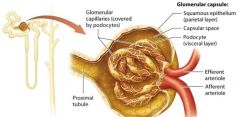
double layered structure that surrounds the glomerulus, consists of an outer parietal layer and an inner visceral layer.
|
|
|
|
The parietal layer |
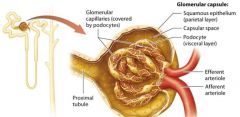
globelike extension of the renal tubule consisting of simple squamous epithelium.
|
|
|
|
The Visceral Layer |
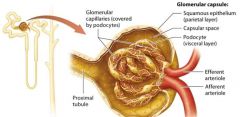
The visceral layer consists of modified epithelial cells called podocytes. |
|
|
|
Podocytes |
wrap around the glomerular capillaries. |
|
|
|
Pedicels |
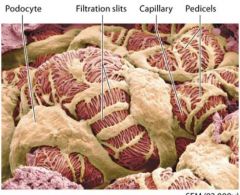
foot processes that extend from each podocyte
Pedicels weave together to form filtration silts. |
|
|
|
Filtration Silts |
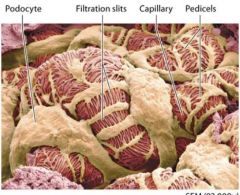
pedicels weave together to form filtration silts which make up another part of the renal corpuscles filtering structure. |
|
|
|
Capsular space |
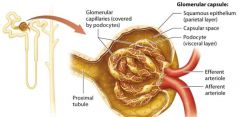
lumen that exists between the parietal and visceral. Is continuous with the beginning of the renal tubule lumen. |
|
|
|
filtering membrane |
The podocytes and fenestrated glomerular capillaries form part of a complex membrane that filters blood flowing through the glomerulus.
This structure allows a large volume of fluid to be filtered from the blood ( which we discuss in the next module). The fluid that passes through the filter to leave the glomerular capillaries, which is known as filtrate, first eneters the capsular space then flows in the renal tubule lumen. |
|
|
|
The Renal Tubule |
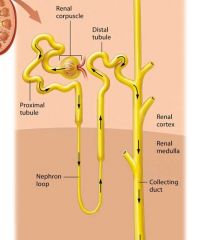
Newly formed filtrate flows from the capsular space into the pipes of the nephron: the renal tubule. The renal tubule is a winding tube responsible for modifying the filtrate. It has three regions
The proximal tubule
The Nephron Loop
The distal Tubule
each differ in structure and function. |
|
|
|
Proximal Tubule |
The initial and longest segment of the renal tubule through which filtrate flows is the proximal tubule; it consists of simple cuboidal epithelial cells with prominent microvilli. This part of the tubule has both convoluted (coiled) and straight sections.
The may microvilli projecting into th lumen of the proximal tubule form a brush border, so named because the fine projections resemble the bristles on a brush. This border greatly increases surface area. |
|
|
|
Nephron loop (loop of Henle) |
The remaining filtrate flows on to the nephron loop, also known as the loop of Henle, which is the only part of the renal tubule that dips into the renal medulla. The nephron loop has two limbs: the descending limb travels toward the renal medulla, turns 180 degrees, and becomes the ascending limb, which climbs back toward the renal cortex. The descending limb is composed of simple squamous epithelium, and thus is often called the thin descending limb. In some nephrons this thin segment also forms the bend region and part of the ascending limb, so there ais also a thin ascending limb. However, the majority of the ascending limb is composed of thicker simple cuboidal epithelium, and is therefore referred to as the thick ascending limb. |
|
|
|
The Distal Tubule |
The final segment through which the filtrate passes in the renal tubule is the distal tubule. Like the proximal tubule, the distal tubule is composed of simple cuboidal epithelium. However the distal fubule lack a brush border. |
|
|
|
The Juxtaglomerular Apperatus |
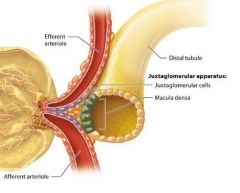
At the transistion point between the ascending limb of the nephron loop and the distal tubule, we find a tightly packed group of cells called the macula densa. The macula densa comes into contact with modified smooth muscle cells in the afferent and efferent arterioles, known as juxtaglomerular (JG) cells.
Together the macula densa and JG cells form a structure called the justaglomerular apparatus (JGA), which regulates blood pressure and glomerular filtration rate. |
|
|
|
The collecting system |
The nephron ends where filtrate in the distal tubule empties into the collecting system, another series of structurally and functionally distinct tubules that further modify the filtrate as it passs through them. Most of the collecting system consists of simple cuboidal or columnar epithelium with few microvilli. |
|
|
|
Components of the Collecting System |
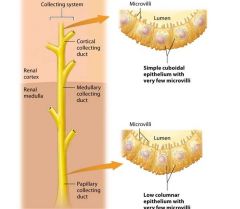
The collecting system consists of the cortical collecting duct and the medullaary collecting system.
The distal tubule empties filtrate into the first part of the collecting system, the cortical collecting duct, which is found within the renal cortex.
The cortical collecting duct is made up of simple cuboidal epithelial cells. Note that each cortical collecting duct drains several distal tubules. As the cortical collecting duct passes into the renal medulla, several medullary collecting ducts empy filtrate into a larger papillary duct. |
|
|
|
The papillary duct |
The papillary duct contains low columnar epithelial cells. The medullary collecting system includes the medullary collecting ducts and the papillary ducts. When the filtrate reaches the end of the papillary duct it is urine. The urine exists at the papillia of the renal pyramid into a minor calyx. The formation of crystals within the tubuls of the collecting system can block the flow of filtrate and lead to intense pain. |
|
|
|
overview |
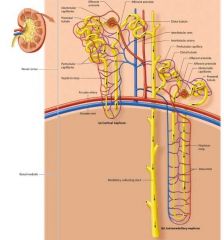
|
|
|
|
Review of Nephron functions |
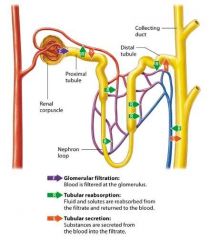
Nephrons carry out three basic physiologic processes that allow the kidneys to perform their homeostatic functions filtration, reabsorption, and secretion. |
|
|
|
Glomerular Filtration |
The first process performe by the nephron is to filter the blood, a process known as glomerular filtration. This takes place as blood passes through the membrane of the glomerular capillaries and some of the plasma is filtered into the surrounding glomerular space. This filter is selective based on size, so it holds back cells and most proteins, which remain in the blood, but allows some of the smaller substances including water, electrolytes (such as sodium and potassium ions) acids and bases (such as hydrogen and bicarbonate ions), organic molecules and metabolic wastes, to exit the blood and enter the glomerular capsule. The fluid and solutes that enter the capsular space of the nephron form the filtrate, also known as tubular fluid.
|
|
|
|
Tubular Reabsorption |
The nect process the nephron performs is to modify the filtrate as it flow through the tubules. Much of this modification involves reclaiming substances from the filtrate, such as water, glucose, amino acids, and electrolytes, and returning them to the blood. This process is known as tubular reabsorption. The nepthron is able to reabsorb the majority of water and solutes filtered by the glomerular membrane. Most reabsorption takes place in the proximal tubule and nephron loop. However some more precisely controlled reabsorption also occurs in the distal tubule and collecting ducts; this process allows the nephron to vary the amounts of different substances reabsorbed to maintain homeostatis as the needs of the body change. |
|
|
|
Tubular Secretion |
The filtrate is also modified by a process known as tubular secretion which is essentially tubular reabsorption in the reverse direction. During tubular secretion substances are moved from the pertibular capillary blood into the filtrate to eventually be exceted. Secretion happens all along the tubule, although different substances may be secreted more in one area than another. Tubular secretion helps maintain electrolyte and acid-base homeostasis and removes toxins from the blood that did not enter the filtrate via filtration. |
|
|
|
The filtration Membrane and the Filtrate |
The inner part of the glomerular capsule consists of three layers that act as barriers, or filters, together these layers are called the filtration membrane. This membrane is made up of the glomerular capillary endothelial cells, a basal lamina, and podocytes (the visceral layer of the glomerular capsule). |
|
|
|
Components of the Filtration membrane |
Fenestrated glomerular capillary endothelial cells Basal lamina Podocytes (visceral layer of the glomerular capsule) |
|
|
|
Fenestrated glomerular capillary endothelial cells |
like all capillaries, the glomeruli are composed of endothelial cells. Recall however that the glomerular endothelial cells are fenestrated they have pores taht make them leakier than most capillaries. The gaps between the glomerular endothelial cells are relativley large (about 70-100 nm) but are still small enough to prevent blood cells and platelets from exiting the capillaries. |
|
|
|
Basal Lamina |
This thin layer of extracellular matrix gel seperates the glomerular endothelial cells from the podocytes. Collagen fibers within the basal lamina form a meshwork that acts like a colander, preventing the capsular space. This effectively blocks the passage of most plasma proteins. In addition the collagen fibers have negative charges that repel negatively charged plasma proteins, even those smaller than 8 nm in diameter. |
|
|
|
Podocytes (visceral layer of the glomerular capsule). |
The podocytes composing the visceral layer of the glomerular capsule make up the third and finest filter in the filtration membrane. Their finger-like pedicels wrap around the glomerular capillaries and interlace to form filtration slits. These narrow slits allow only substances witha diameter less than 6-7 nm to enter the capsular space. Albumin the moste prevelent plasma protein, has a diameter of 7.1 nm, so its is normally prevented from entering the filtrate. |
|
|
|
Functions of the filtration membrane |
The fluid and solutes that pass through the filtration membrane and enter the capsular space make up the filtrate. The size of the pores in this membrane determins the composition of the filtrate.
The filtration membrane allows water and small dissolved solutes, including glucose, electrolytes, very small proteins, and amino acids, to leave the blood and enter the capsular space, but prevents the exit of formed elements, such as cells and platelets, and most proteins. Another importnt substance filtered from the blood is a group of molecules known as nitrogenous wastes. These molecules include urea and ammonium ions (NH4+), waste products of protein metabolism; creatine, a waste product of the creatine kinase reaction that occurs in muscle cells and other body cells; and uric acid, a waste product of nucleic acid metabolism. All are relatively small molecules that pass easily through the pores of the filtration membrane.
The percentage of plasma that passes through the filtration membrane to enter the capsular space and become filtrate is called the filtration fraction. An average filtration fraction is 20% which means that about one-fifth of the plasma that flows through the glomerulus exits the blood and enters the capsular space. The filtration fraction is so large becuase of the looping arrangement of glomerular capillaries. This looping increases their surface area dramatically if you were to stitch one kidney together, their combined area would be about 6 m^2 (about the size of a small bedroom). The large surface area makes filtration through this stack of ever finer filters a very efficient process.
|
|
|
|
The glomerular FIltration Rate (GFR) |
Filtrate is formed at the remarkably rapid rate of about 125 ml/min. This value, the amount of filtrate formed by both kidneys in 1 minute, is known as the glomerular filtration rate GFR. Over the course of a day, the kidneys produce about 180 liters of filtrate. Your entire plasma volume is filtered by your kidneys about 60 times per day. The kidneys are able to filter blood so efficiently in part because the glomerular capillaries are remarkably permeable. However, even with fenestrated capillaries, filtration will happen only if a pressure gradient is present to push water and solutes through the filtration mebrane. |
|
|
|
Filtration Pressures |
Hydrostatic Pressure
Colloid osmotic pressure |
|
|
|
Hydrostatic Pressure |
Hydrostatic pressure is the force of a fluid on the wall of its container. In the case of blood capillaries, hydrostatic pressure is equal to the blood pressure, and it tends to push water out of the capillary and into the intersitial space. |
|
|
|
Colloid osmotic pressure |
Colloid osmotic pressure (COP) is the pressure created by proteins (Primarliy albumin) in the plasma. The osmotc gradient created by these proteins pulls water into the capillaries by osmosis. |
|
|
|
Net Filtration Pressure |
Hydrostatic pressure and Colloid Osmotic Pressure work together in the capillary bed to determine the net filtration pressure (NFP) of the bed. The net filtrattion pressure determines the direction of water movement betwwen the capillaries and the interstitial fluid. Simply stated, water moves out of the capillary if hydrostatic pressure is higher than COP, or into the capillary if COP is higher than hydrostatic pressure. |
|
|
|
Net Filtration Pressure at the Glomerulus |
The situation is slightly more complex in the glomerulus, because we have a third force to factor in: the hydrostatic pressure of the fluid in the capsular space. |
|
|
|
Forces in the glomerulus |
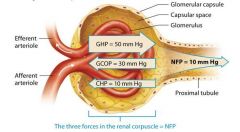
Glomerular hydrostatic pressure
Glomerular Colloid Osmotic Pressure
Capsular Hydrostatic Pressure |
|
|
|
Glomerular Hydrostatic Pressure (GHP) |
Glomerular hydrostatic pressure (GHP), which is largely determined by the systemic blood pressure, measures about 50 mm Hg. This pressure is considerably higher than that of a typical capillary bed ( which ranges from 17 to 35 mm Hg). This is because blood leaving such a capillary bed enters a low resistance venule, whereas blood leaving the glomerulus enters a high resistance efferent arteriol. This arterioles high resitance is due to its smooth muscle and its small diameter. The diameter of the efferent arteriole is smaller than that of the afferent arteriole. This causes blood to back up and push against the walls of the glomerular capillaries, which favors its movement through the filtration membrane. |
|
|
|
Glomerular Colloid Osmotic Pressure |
Like the COP in typical capillaries, the glomerular colloid osmotic pressure (GCOP) is created by the presence of proteins such as albumin in the plasma. The GCPOP averages about 30 mm Hg, slightly higher than the COP in a typical capillary bed, because the blood in the glomerulus is a bit more concentrated. The reason for this is that water leaves the glomerular blood rapidly through the filtration membrane, which cause any solutes left in the blood to increase in concentration. The GCOP opposes filtration, "pulling" on water to hold it in the glomerular capillaries. |
|
|
|
Capsular Hydrostatic Pressure |
Like an emptying kitchen sink with the faucet turned on, the water in the capsular space can only drain into the renal tubule so quickly. The rapidly accumulating filtrate inside the capsular space of a nephron builds up a hydrostatic pressure of its own, called the capsular hydrostatic pressure (CHP). This pressure ( about 10 mm Hg) tries to push water into the gloerular capillaries and so opposes filtration. |
|
|
|
Net filtration pressure |
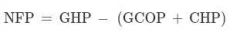
We can find NFP using Glomerular Hydrostatic Pressure, Glomerular Colloid Osmotic Pressure, Capsular Hydrostatic Pressure. The total pressure gradient available to drive water across the filtration membrane and into the capsular space. To find the glomerular NFP, we subtract the two forces that oppose filtration (GCOP and CHP) from the one that favors filtration (GHP) |
|
|
|
Glomerular Filtration Rate |
The net filtration pressure combined with the leakiness of the glomerular capillaries and their large surface area gives the Glomerular Filtration Rate. The GFR determine how rapidly the blood is cleansed of metabolic wastes, how effectively the kidneys can carry out both tubular reabsorption and secretion, and how well the kidneys are able to maintain homeostasis in the body. For these reasons, factors that impact the GFR influence all functions of the kidney. |
|
|
|
Factors that affect the Glomerular Filtration Rate |
Autoregulation within the kidney itself
neural and hormonal factors |
|
|
|
Autoregulation of the GFR |
autoregulation of the GFR consists of two negative feedback processes: the myogenic mechanism and tubuloglomerular feedback. |
|
|
|
The Myogenic Mechanism |
An increase in blood pressure stretches a blood vessel, which triggers its smooth muscle cells to vasoconstrict. This decreases the amount of blood flowing through the vessel, which in turn minimizes the stretch imposed on the vessel. We find a similar phenomenon in the blood vessels of the kidney, which is termed the myogenic mechanism.
The myogenic mechanism acts rapidly it can restore the normal GFR within second of even a significant change in blood pressure. Note, however, that these mechanism can restore the GFR over a systolic blood pressure range of about 80-180 mm Hg. If the systolic blood pressure goes higher than 180 mm Hg, the affernet arteriole can't constrict any further to restore the GFR. Similarly, if systolic blood pressure drops below this range, that afferent arteriole is unable to dilate enough to restore the GFR to normal levels.
|
|
|
|
increases in systemic blood pressure |
An increase in systemic blood pressure stretches the afferent arteriole and leads to an increase in GFR. This leads the muscle cells to contract, constricting the arteriole. Vasoconstriction of the afferent arteriole decreases the blood flow through the glomerulus, which decreases glomerular hydrostatic pressure and the GFR back to normal levels. |
|
|
|
Decreases in systemic blood pressure |
A decrease in the systemic blood pressure causes the afferent arteriole to be less stretched, decreasing the GFR and making its smooth muscle cells relax. The resulting vasodilation of the arteriole increases the blood flow and the glomerular hydrostatic pressure, and the GFR increases back to normal levels. |
|
|
|
Tubuloglomerular Feedback |
The second autoregulatory mechanism of the kidneys is tubuloglomerular feedback, so named because the macula densa of the distal renal tubule is part of a negative feedback loop that controls pressure in the glomerulus. As its currently understood, the mechanism of tubuloglomerular feedback is related to the concentration of NaCl in the filtrate. The basic feedback loop proceeds by the following steps: If the GFR increases, the volume of filtrate flowing through the renal tubule increases. The increased filtrate volume makes the filtrate flow more rapidly through the tubules. This leads to an increased delivery of sodium and chloride ions to the macula densa cells in the distal tubule, causing them to absorb more of these ions from the filtrate. In an example of the Cell-Cell Communication Core principle, the macula densa cells release chemicals that diffuse through the interstitial fluid to the afferent arteriole, which they trigger to constrict. The macula densa cells signal the renin containing JG cells to reduce their release of renin, leading to a decreased renal production of angiotensin-II and dilation of the efferent arteriole. The GFR decreases back toward normal |
|
|
|
Decreases in GFR |
A decrease in GFR is thought to have the opposite effect fewer sodium and chloride ions are delivered to the macula densa cells, causing the afferent arteriole to dilate and the efferent arterioles to constrict. This increases the glomeruar hydrostatic pressure and restores the GFR.
|
|
|
|
myogenic mechanism & tubuloglomerular feedback cooperation |
With both the myogenic mechanism and tubuloglomerular feedback at work, the GFR remains remarkably consistent through changes in blood pressure, which happern with some frequency. For example every time you sneeze your blood pressure changes momentarily. |
|
|
|
Greater changes in blood pressure |
if systolic blood pressure rises above 180 mm Hg, the GFR and urine output will increase dramatically. Conversely if systolic blood pressure drops below about 70 mm Hg, glomerular filtration will cease becuase the compensatory mechanisms cannot create NFP that favors filtration. At this point, no urine wil be produced, a life threatening condition called anuria. |
|
|
|
Hormonal Effects on the GFR |
Hormones that affect the GFR do so by adjusting the glomerular hydrostatic pressure. Hormones also affect the GFR as part of a larger system that regulates systemic blood pressure. One hormone that regulates the GFR is angiotensin II, a component of the renin angiotensin aldosterone system. Another set of regulating hormones consists of the natriuretic peptides produced by the heart. |
|
|
|
The renin Angiotensin Aldosterone System |
The renin angiotensin aldosterone system RAAS is a complex system whose primary function is to maintain systemic blood pressure; it preserves the GFR as a secondary effect. Therefore it acts on much more than just the afferent and or efferent arterioles of the glomeruli. The RAAS also significantly impacts tubular reabsorption in the nephron and collecting system in order to influence electrolyte balance and blood volume, in addition to causing changes within the body as a whole.
This system may be triggered into action by three conditions
stimulation from neurons of the sympathetic nervous system
low glomerular hydrostatic pressure
stimulation from the macula densa cells as part of tubuloglomerular feedbacck. The RAAS maintains blood presure over both the short and long term. The vasoconstriction produced by A-II is nearly immediate, blood pressure rises whithin second of exposure to A-II. However, this vasoconstriction lasts only 2-3 minutes. Long-term blood pressure control comes from the retention of sodium ions and water from the filtrate. Notice that the effects of the RAAS on both vasoconstriction and sodium ion reabsoption allow the body to increase systemic blood pressure while preserving the GFR. In addition to its effects on blood pressure, the RAAS is also critical to maintaining sodium ion balance. When the sodium ion level decreases in the plasma, and so in the filtrate, the macula densa cells stimulate renin secretion from the JGA cells, and more sodium ions are reabsorbed into the bllod from the filtrate in the renal tubules. Whn the plasma sodium ion level increases, renin secretion decreases, and the kidneys excrete more sodium ions. The RAAS is so effective at this task that sodium ion intkae can increase up to 50 times nad the level of sodium ions in the plasma will remain relatively unchanged. |
|
|
|
Steps of the RAAS |
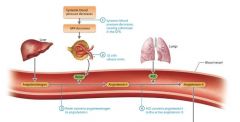
Systemic blood pressure decreases causing a decrease in the GFR
JG cells release renin. Decreased blood flow through the afferent arteriole triggers the JG cells to release the enzyme renin into the bloodstream.
Renin converts angiotensinogen to angiotensin-I. Renin circulates until it encounters an inactive protein produced by the liver called angiotensinogen. Renin catalyzes the conversion of angiotensinogen to a product with minimal activity, angiotensin-I (A-I).
ACE converts angiotensin-I to the active angiotensin-II. A-I circulates through the blood until it encounters an enzyme called angiotensin converting enzymes ACE, which is made by cells as the endothelial cells in the lungs. ACE converts A-I to its active form Angiotensin-II (A-II). |
|
|
|
A-II effects on the systemic blood pressure and the GFR |
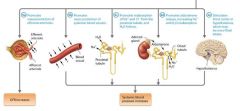
Promotes vasoconstriction of efferent arterioles
Promotes vasoconstriction of systemic blood vessels
Promotes reabsorption of sodium and chloride ions from the proximal tubule, and water follows.
Promotes aldosterone release, leading to increased sodium ion and water reabsorption
Stimulates thirst |
|
|
|
Promotes Vasocontriction of Efferent Arterioles |
A-II constricts all of the renal blood vessels, but it has a greater effect on the efferent arterioles than on the afferent arterioles. This "Clogs the drain", raising glomerular hydrostatic pressure. This increased pressure, in turn, increases the GFR to maintain the filtration rate despite the reduced blood floe. |
|
|
|
Promotes Vasoconstriction of systemic blood vessels |
A-II is a powerful vosoconstrictor of nearly all systemic vessels (it causes effects more than 40 times stronger than those of norepinephrine). This vasoconstriction increases peripheral resistance, which in turn increases systemic blood pressure |
|
|
|
Promotes reabsorption of sodium and chloride ions from the proximal tubule, and water follows |
One of the most powerful effects of A-II on blood pressure comes from its role in renal tubule reabsorption. In the proximal tubules, A-II promotes reabsorption of both sodium and chloride ions from the filtrate, which in turn causes reabsorption of water by osmosis. This increases blood volumes, which raises blood pressure. |
|
|
|
Promotes aldosterone release, leading to increased sodium ion and water reabsorption |
A-II stimulates the adrenal glands to release the hormone aldosterone. Aldosterone acts on the distal tubule and parts of the collecting system to increase sodium ion reabsorption from the filtrate. If ADH is present, water follows by osmosis, so this action increases blood volume, and therefore blood pressure. |
|
|
|
Stimulates Thirst |
Another effect of A-II is to stimulate the thirst centers in the hypothalamus. This can increase fluid intake, which would increase blood volume. |
|
|
|
Atrial Natriuretic Peptide |
Certain cells of the atria in the heart produce the hormone Atrial Natriuretic Peptide ANP. ANP, which is released when the volume of blood in the atria increases, acts to lower blood volume and blood pressure and thereby reduve the workload of the heart. One of the ways that ANP accomplishes this task is by increasing the GFR. ANP dilates the afferent arterioles and constricts the efferent arterioles of the glomeruli, a combination that "turns up the fuacet" and "clogs the drain". This increases the glomerular hydrostatic pressure raising the GFR. High GFR leads to more fluid loss and therefore decreases blood volume and effectively lowers blood pressure. |
|
|
|
Neural Regulation of the GFR |
Neural control of the GFR is chiefly mediated by the sympathetic division of the autonomic nervous system. As with the hormonal systems just described, the sympathetic nervous system affects the GFR as part of a larger system to control blood pressure. Sympathetic neurons release norepinephrine during times of increased sympathetic activity, which causes constriction of most systemic blood vessels, including the afferent arterioles, and so elevates systemic blood pressure. You might think this should decrease glomerular hydrostatic pressure and the GFR, but the sympathetic effect on the GFR isnt quite that simple. Instead, its impact depends on how much it is stimulated. |
|
|
|
low levels of sympathetic stimulation |
If the level of sympathetic stimulation is low (eg during mild exercise such as walking) sympathetic neurons trigger the JG cells to release renin. This leads to the formation of a low level of A-II, which raises systemic blood pressure and increases the GFR. |
|
|
|
High levels of sympathetic stimulation |
If the level of sympathetic stimulation is high (eg during strenous exercise or severe blood loss) a large amount of renin is secreted, and the blood concentration of A-II increases dramatically. A high level of A-II will actually constrict both the afferent and efferent arterioles of the glomeruli, decreasing the filtration rate. This is especially important in cases of severe hypotension and dehydration, as it helps the body to minimize fluid loss and preserve blood volume and blood pressure. In these cases, perfusion of muscle tissue and vital organs (brain, heart) takes precedence over filtration in the kidneys. |
|
|
|
Renal Failure |
Occurs when a person GFR decreases so that the kidneys are unable to carry out their vital functions. Many conditions may lead to renal failure, including factors that decrease bllod flow to the kidneys, diseases of the kidneys themselves and anything that obstructs urine outflow from the kidneys. Short term renal failure which is known as acute renal failure or acute kidney injury is common among hospitalized patients and may resolve completely with treatment of underlying cause. Some people develop chronic long term renal failure, which is defined as a decrease in GFR lasting 3 months or longer. The biggest risk factors for the development of chronic renal failure are diabetes mellitus and hypertension. Symptoms depend on the severity of the renal failure, and those with mild renal failure might not notice any symptoms. As renal function declines, patients experience fatigue, edema, nausea, and loss of apetite. Severe renal failure, in which the GFR is less than 50% of normal, results in a condition known as uremia. |
|
|
|
Uremia |
the buildup of waste products and fluid, electrolyte, and acid base imbalances. Can be fatal |
|
|
|
dialysis |
two types
hemodialysis which temporarily removes an individuals blood and passes it through a filter that removes metabolic wastes and extra fluid, and normalizes electrolyte and acid base balance. Hemodialysis must be performed three times per week at a dialysis clinic.
Pertonial dialysis is when fluid is placed into the peritoneal cavity, allowed to circulate for several hours and then drained. A patient is able to undergo peritoneal dialysis nightly at home, so this is often the preferred treatment for individuals needing long term dialysis. |
|
|
|
Tubular Reabsorption and Secretion |
Because of the high rate of filtrate formation, the kidneys have to work to reclaim most of the filtrate and return it to the bloodstream. Recall that this occurs by the process of tubular reabsorption in the nephrons and collecting ducts. Of the 180 liters of water filtered by the kidneys every day only about 1.8 liters ultimately leave the body as urine, which means that kidneys are very efficient at reclaiming water. In addition, about 99% of the solutes filtered are subsequently reabsorbed. Given the magnitudes of these quantities, its not surprising that the kidneys use approximately 20% of the bodys ATP at rest. Since our kidneys reabsorb so much of what they filter, you may wonder why our bodies need to filter blood through the kidneys at all. First of all, certain waste products are not reabsorbed. In addition in spite of the high GFR some waste substances do not pass through the filtration membranes of the glomeruli and therefore dont enter the filtrate. This necessitates the process of tubular secretion, which occurs in the renal tubules and collecting ducts at the same time as tubular reabsorption. Certain substances are secreted into the filtrate these nonreabsorbed and secreted substances are often toxic when present in high concentrations, and the high GFR and process of secretion ensure their rapid clearance from the body. In addition the presence of a large volume of filtrate in the nephrons gives the kidneys precise contorl over homeostasis of fluid, electrolyte, and acid-base balance. If substances are not needed, they are excreted with the urine. If they are needed, they will be reabsorbed into the blood. |
|
|
|
Transport of substances |
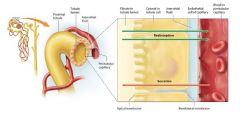
in tubular reabsorption substances must pass from the filtrate in the lumen of the tubule across or between the tubule cells, into the intersitial fluid, and finally across or between the endothelial cells of the peritubular capillaries to re-enter the blood. In tubular secretion, substances move in the opposite direction. |
|
|
|
Paracellular Route |
On the paracellular route, substances pass between adjacent tubule cells. The tight junctions between the tubule cells are just leaky enouggh to allow some substances such as small ions and water to move passively between them, particularly in the proximal tubule.
Transport along the paracellular route is passive, requireing no energy in the form of ATP, becuase susbtances move with their concentration gradients via diffusion or osmosis. The same is true for certain substances taking the transceullar route. However, other substances moving along the transcellular rout travel against their concentration gradients and thus require energy either directly or indirectly, from ATP. Secretion is an active proces, so it must occur via the transcellular route across the tubule cell membrane.
Reabsorbed substances that have entered into the interstitial fluid may then cross the endothelial cells of the blood vessel and enter the blood. These substances can follow the same routes into the capillary that they followed to exit the tubule they may take the paracellular rout or the transcellular route. Generally, these processes are passive and solutes move by diffusion and water by osmosis. |
|
|
|
Transcellular Route |
On the transcellular route substances such as glucose and amino acids must move through the tubule cells. A reabsorbed substance first crosses the apical membrane of the tubule cell ( the membrane facing the tubule lumen) then travels through the cytosol, and finally exits the cell through the basolateral membrane. |
|
|
|
Carrier Mediated Transport |
most of the substances that are reabsorbed and secreted via the transcellular route require the use of a carrier protein in the tubule cell plasma membrane. Recall that there are three ways in which cells use carrier proteins to transport substances.
Facilitated diffusion- in which a carrier protein passively transport a substance with its concentration gradient, without using energy from ATP.
Primary Active Transport- In which a carrier protin Pump directly uses ATP to move a substance against its concentration gradient
Secondary active Transport- In which a concentration gradient set up by a primary active transport pump is used to drive the transport of a second substance against its concentration gradient via another carrier protein. |
|
|
|
Antiporters & Symporters |
There are two types of active transport carrier proteins Antiport pumps (anitporters) move two or more substances in opposite directions, and symport pumps ( or symporters) move two or more substances in the same direction. Both have a limited number of sites on which they can transport substances, much as a train only a certain number of seats for passegners. If all of thier sites become filled, the carrier proteins are said to be saturated, as they have reached their transport maximum (Tm). Any substances unable to bind to their carrier proteins will likely not be transported and will end up in the urine. This is what happens to glucose in diabetes mellitus. |
|
|
|
Reabsorption and Secretion in the Proximal Tubule |
The cells of the proximal tubule have prominent microvilli that provide these cells with a large surface area. This facillitates the remarkably rapid reabsorption that ocurs in this very active segment of the renal tubule. In fact the proximal tubule is the most metabolicially active part of the nephron, as most of the filtrate is reabsorbed here its Na/K pumps alone consume about 6% of the body's ATP at rest. In addition to all of this reabsorption a great deal of secretion takes place in the proximal tubule as well. Thee |
|
|
|
Roles of the proximal tubule in reabsorption |
reabsorption of a large percentage of electrolytes, including sodium, chloride, potassium, sulfate, and phosphate ions, an acitivity that is vital for electrolyte homeostasis reabsorption of nearly 100% of nutrients such as glucose, amino acids, and other organic substances lactic acid and water soluble vitamins reabsorption of many of the bicarbonate ions, which is critical for acid base homeostasis reabsorption of about 65% of the water, which is required for maintenance of the body's fluid homeostasis |
|
|
|
Reabsorption of Sodium Ions in the proximal tubule |
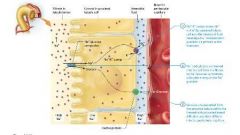
The majority of sodium ion reabsorption occurs through sodium ion leak channels on the apical surface of the proximal tubule cell, driven by its concentration gradient.
During active transport by the transcellular route, the cells of the proximal tubule have three types of carrier proteins for sodium ions in their apical membranes:
Carrier proteins specific for sodium ions that enable facilitated diffusion of sodium ions from the filtrate into the tubule cells
Na+ symporters that bring sodium ions from the filtrate into the cells with other solutes such as glucose
Na+/H+ antiporters that bring sodium ions into the cells while secreting hydrogen ions into the filtrate
Each of these three carrier proteins transports sodium ions down its concentration gradient into the tubule cell. This concentration gradient isnt present naturally it is created by Na+/K+ pumps in the basolateral membrane that continually pump sodium ions out of the tubule cells and into the interstitial fluid. These pumps create a relatively low sodium ion concentration in the proximal tubule cells, whereas the sodium ion concentration in the filtrate is higher ( about 142 mOsm). This concentration gradient is critical for the secondary active transport of many other solutes.
Most activity in the proximal tubule occurs without any outside control. Exceptions occur, such as parathyroid hormone decreasing phosphate ion reabsorption. In addition, the Na+/K+ pumps in the basolateral membrane become more active in the presence of angtiotensin-II, which explains how this hormone increases sodium ion reabsorption from proximal tubule cells. |
|
|
|
Reabsorption of Organic Solutes and Ions |
The cells of the first half of the proximal tubule contain both Na+/K+ pumps and Na+/glucose symporters. The symporters use the sodium ion gradient created by the pumps to carry both glucose and sodium ions from the filtrate into the tubule cell, and example of secondary active transport. Once in the cell glucose is transported via facilitated diffusion into the interstitial fluid, where it diffuses into the peritubular capillaries. Other symporters in the apical membrane of the proximal tubule cells function in a similar fashion, allowing the secondary active transport of sodium ions and another solute, such as anion SO4 2-, HPO 4 2-, or an organic solute such as amino acids or lactic acid. The reabsorption of sodium ions also leads to the reabsorption of anions such a chloride in another way too. As sodium ions are passivley transported out of the tubule lumen, the lumen accumulates a net negative charge. This creates an electrical gradient that pushes the negatively charged chloride ions across the epithelium through the paracellular route. These chloride ions similaraly follow sodium ions into the interstitial fluid and into the plasma, as well. |
|
|
|
Bicarbonate Ion Reabsorption |

Bicarbonate ion reabsorption from the proximal tubule involves principles that you learned in the chemistry chapter about carbonic acid-bicarbonate buffer system. Recall that carbon dioxide (CO2) in the blood reacts with water (H2O) to produce carbonic acid (H2CO3) this reaction is catalyzed by the enzyme carbonic anhydrase (CA). Newly formed carbonic acid spontaneously dissociates into bicarbonate (HCO3) and hydrogen (H+) ions.
Bicarbonate reabsorption from the proximal tubule occurs in a roundabout way. The proces somewhat resembles a game of fetch. The cell "tosses" hydrogen ions into the filtrate, and the hydrogen ion "fetches" a bicarbonate ion, which is then brought back into the cell. This toss is accomplished with the help of another carrier protein: the Na+/H+ antiporter. This carrier protein transports sodium ions into the cell while secreting hydrogen ions from the cell into the filtrate. |
|
|
|
Na+ / H+ antiporter carrier protein process |
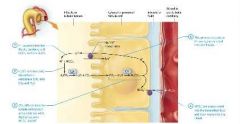
Hydrogen ions secreted into the filtrate combine with bicarbonate ions to form carbonic acid. Hydrogen ions are secreted from the cytosol of the proximal tubule cell into the filtrate by the Na+/H+ antiporter. The H+ react with HCO3- in the filtrate to form H2CO3. Carbonic acid is converted via carbonic anhydrase to carbon dioxide and water. Carbonic anhydrase on the apical plasma membrane of the tubule cell catalyzes the conversion of H2CO3 into the CO2 and H2O. Carbon dioxide diffuese into the tubule cell cytosol and combines with water to become bicarbonate and hydrogen ions. CO2 diffuses into the cytosol of the tubule cell with its concentration gradient. Carbonic anhydrase within the cell catalyzes the conversion of CO2 and H2O into the H2CO3, which then dissociates into HCO3- and H+. Bicarbonate ions are transported into the interstitial fluid and then move into the blood. The HCO3- is transported across the basolateral membrane to enter the interstitial fluid nad then diffuses into the peritubular capillaries. The process repeats as hydrogen ions are again secreted into the filtrate. Hydrogen ions are again secreted into the filtrate by Na+/H+ antiporter, and the process is repeated. Though this process may seem complicated it is effective and allows the cells of the proximal tubule to reabsorb about 90% of the bicarbonate ions from the filtrate. This is a key component of the body's ability to maintina the pH of the blood within a very specific range. |
|
|
|
Obligatory Water Reabsorption and Its Effect on Other Electrolytes |
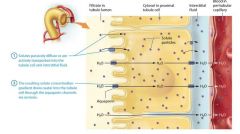
By the time the filtrate has reached the second half of the proximal tubule, many of the sodium ions as well as glucose and other organic molecules have been reabsorbed. This creates a gradient favoring the passive movement of water by osmosis out of the filtrate along both the paracellular and transceullar routes. Remember that in osmosis, water moves to the solution with a higher solute concentration. This type of water reabsorption is called obligatory water reabsorption, becuase water is obliged to follow solute movement. A kind of water channel in the plasma membrane called an aquaporin greatly enhances rapid water reabsorption. These channels, which are located in both the apical and basolateral membranes of proximal tubule cells, allow water to move through these cells via the transcellular route. As obligatory water reabsorption continues, the conentration of solutes, such as potassium, calcium, and magnesium ions, rises the filtrate. This creates a concentration gradient that favors their diffusion into or between the proximal tubule cells. Active reabsorption of solutes stimulates further reabsorption of water by osmosis. This turn stimulates passive reabsorption of other solutes. |
|
|
|
Secretion in the Proximal Tubule |
In addition to the hydrogen ion secretion we discussed earlier, other subtances are secreted into the filtrate by the proximal tubule cells, including many nitrogenous waste products and drugs. In the first half of the proximal tubule, most of the uric acid in the filtrate is reabsorbed, but nearly all of it is secreted back into the filtrate in the second half of the tubule. Additionally, ammonium ion NH4+, creatine and small amounts of urea are also secreted. Drugs such as penicillin and morphine have significant renal secretion. These must be taken ofter because medicine lost through renal secretion must be replaced in order to maintain relatively consistent blood levels. |
|
|
|
Reabsorption in the Nephron Loop |
By the time filtrate reaches the nephron loop it barely resembles the original filtrate about 60-70% of electrolytes and water have been reabsorbed from the filtrate and returned to the blood, in addition to most of the organic solutes such as glucose and amino acids. As the filtrate flows through the nephron loop it undergoes further losses: Approximately 20% of the total water, 25% of the total sodium and chloride ions, and a significant portion of the remaining ions are reabsorbed and returned to the blood. In the proximal tubule you saw that water reabsorption is proportional to solute reabsorption. For this reason, the filtrate in the proximal tubule has the same concentration or osmolarity as the interstitial fluid, about 300 mOsm. In the nephron loop however, the filtrates osmolarity changes as it flow through the loop. This is due to the differing permeabilities in the ascending and descending limbs of the nephron loop. The thin descending limb of the nephron loop is freely permeable to water, but much less permeable to solutes such as sodium and chloride ions. So water can move out of the thin descending limb cells by osmosis, but few solutes follow. This causes the osmolarity of the filtrate to increase as it passes down the descending limb and rounds the bend of the loop. The cells of the thick ascending limb are impermeable to water, but they transport NaCl into the tubule cells with the use of Na+/K+/2Cl- symporters. This secondary active transport system brings one sodium, one potassium, and two chloride ions into the tubule cell, relying on the Na+/K+ pump on the basolateral membrane to create a favorable sodium ion gradient. Remember that the Na/K+ pumps drive potassium ion back into the tubule cell so ther isnt much net reabsorption of potassium ions in this process. As filtrate passes through the ascending limb, it loses solutes and gradually becomes less concentrated as ions are pumped into the interstitial fluid. These differing permeabilites in the two limbs and the changing concentration of the filtrate are part of a larger system that allows for extensive water reabsorption from the filtrate in both the loop and the collecting system. |
|
|
|
Reabsorption and Secretion in the Distal Tubule and Collecting System |
By the time the filtrate enters the first part of the distal tubule about 85% of the water and 90% of the sodium ions have been reabsorbed. For this reason, the rate of filtrate flow in this part of the tubule is signigicantly lower about 20 ml/min than it was in the early proximal tubule about 120 ml/min. The cells of the distal tubule lack microvilli. Most of the water reabsoption has already taken place and so the cells do not require microvilli to perform their function of reabsorbing much of the remaining water and solutes. Even though so much reabsorption has taken place, if we excreted the remaining water and sodium ions in the urine, we would still lose about 29 liters of water and a significant protion of our sodium ions every day. This situation would be incompatible with life. Reabsorption of the remaining water and sodium ions, then, is still critical. The early distal tubule is structurally and functionally similar to the ascending limb of the nephron loop. However the latter portion of the distal tubule is very similar to the cortical collecting duct, so we discuss them together here. Then we examine the medullary collecting system, which differs structurally and functionally from these other areas. |
|
|
|
The late Distal tubule and cortical collecting Duct: Hormone regulation |
Cells of the late distal tubule and cortical collecting duct have hormone receptors that determine their function; the number and types of these receptors vary with hormone stimulation. The majority of their activity is therefore regulated by hormones in order to fine tune water, electrolyte, and acid base balance. For this reason, the water reabsorption here is called faculative water reabsorption, because water is reabsorbed in accordance with the bodys needs. The hormones involved in faculative water reabsorption as well as water and electrolyte balance include the following Aldosterone Antidiuretic Hormone Atrial Natriuretic Peptide (ANP) |
|
|
|
Aldosterone |
Aldosterone is a steroid hormone synthesized and released by the adrenal cortex that interacts with the DNA of cells to increase their permeability to sodium ions and the number of thier Na+/K+ pumps. Both action increase the reabsorption of sodium ions from the filtrate and the secretion of potassium ions inot the filtrate. Note that these actions also indirectly cause reabsoption of water and chloride ions, becuase as sodium ions are reabsorbed, water and chloride ions passively follow (if antidiuretic hormone is present). Aldosterone also stimulates secretion of hydrogen ions into the filtrate by specialized cells called intercalated cells.
|
|
|
|
Antidiuretic Hormone |
Recall that antidiuretic hormone ADH is amde by the hypothalamus and released form the posterior pituitary gland. Note that diuresis refers to loosing body water to the urine, and a diuretic is an agent that promotes diuresis. An anti-diuretic therefore refers to an agent that causes water retention and reduces urine output. ADH exerts these effects by causing aquaporins to be inserted into the cells apical membranes, permitting rapid water reabsoption. In the absence of ADH the cells of the late distal tubule and cortical collecting duct are barely permeable to water, and a lerger volume of water is lost in the urine. |
|
|
|
Atrial Natriuretic Peptide (ANP) |
ANP triggers natriuresis or urinary excretion of sodium ions. It also appears to inhibit release of ADH and aldosterone, causing fewer sodium ions ( and also less water) to be reabsorbed, and so more sodium ions and water to appear in the urine. |
|
|
|
The Medullary collecting System |
As filtrate flow through the medullary collecting ducts and the papillary ducts, this is the kidneys last chance to regulate fluid, electrolyte, and acid base balance before the filtrate becomes urine. |
|
|
|
Cells of the medullary Collecting system |
They are impermeable to water in the absence of ADH. However, in the presence of ADH, they reabsorb large volumes of water. They are permeable to urea, which allows some urea to move down its concentration gradient into the intersitial fluid. Intercalated cells in this region actively secrete hydrogen ions from the interstitial fluid into the filtrate against a very high concentration gradient;jj they can increase the concentration of hydrogen ions in the filtrate about 900 times. |
|
|
|
Acid Base maintenance of tubular reabsorption and secretion |
Variable reabsorption and secretion of hydrogen and bicarbonate ions contribute to pH homeostasis o f the extracellular fluids, including blood. Thus far, you have seen two examples of acid-base regulation throghout the renal tubule and collecting system; The cells of the proximal tubule secrete hydrogen ions as a way to reabsorb bicarbonate ions, and the intercalated cells of the late distal tubule and collecting system actively secrete hydrogen ions under the influence of aldosterone. Another mechanism in renal tubule cells is stimulated when the pH of the blood becomes abnormal. If the pH of the blood decreases making it too acidic enzymes in the tubule cells will remove the amino group from the amino acid glutamine in the cytosol. In doing this, the cells generate two ammonia molecules (NH3) and two bicarbonate ions. The ammonia is then secreted and the bicarbonate ions are reabsorbed. In thej filtrate, the ammonia molecules bing and bufferr hydrogen ions to form the ammonium ion NH4. Both ammonia buffering and bicarbonate ion reabsorption help to raise the pH o the blood back to normal. If the pH of the blood increases making it too alkaline, the tubule cells will reabsorb fewer bicarbonate ions from the filtrate, excreting them in the urine and lowering the pH of the blood. |
|
|
|
The Big Pictures of tubular Reabsorption and Secretion |
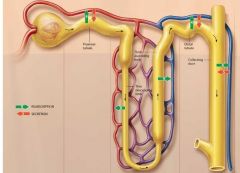
|

|
|
|
Regulation of Urine Concentration and Volume Faculative reabsorption |
ABout 85% of water reabsorption in the kidney is obligatory, water is obliged by osmossis to follow solutes that have been reabsorbed. The last 15% of water reabsorption is faculative water reabsorption, which is adjusted by hormones to meet the body's needs and maintain fluid homeostasis. Faculative water reabsorption is what determines final urine concentration and volume. |
|
|
|
Osmolarity of the Filtrate entering the proximal renal tubule |
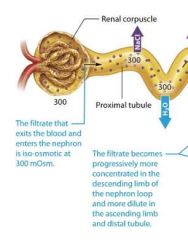
The filtrate that exits the blood and enters the renal tubule initially is iso-osmotic or equally osmotic, to the plasma at 300 mOsm. |
|
|
|
Osmolarity of the filtrate in the nephron loop |
In the nephron loop the filtrates osmolarity changes because of the differing permeabilites of its ascending and descending limbs. |
|
|
|
The thin descending limb |
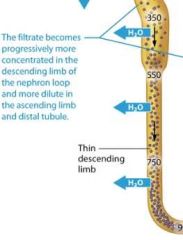
The thin descending limb of the nephron loop is permeable to water but not solutes, as you learned, so water flows from the filtrate to the intersitial fluid by osmosis, but very few solutes follow. This causes the filtrate to become progressively more concentrated as it travels down the loop. By the time it reaches the bottom of the loop, on average it is approximately 900 mOsm, about three time more concentrated than plasma. |
|
|
|
The thick ascending limb |
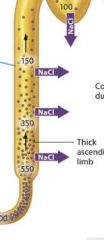
As the filtrate enters the thick ascending limb, sodium and other ions are pumped out of the filtrate and into the interstitial fluid. However because this part of the limb is virtually impermeable to water, water cant follow these solutes. So the concentration of the filtrate decreases as it moves up the ascending limb of the loop. By the time the filtrate exits the thick ascending limb and moves into the distal tubule, its osmolarity is generally less than that of filtrate at the same level in the thin descending limb. |
|
|
|
The early distal tubule |
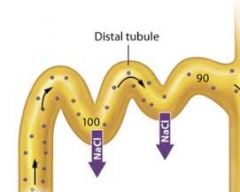
In the early distal tubule ions continue to leave the filtrate while water stays behind, and the concentration of the filtrate decreases even further to about 100 mOsm, or three time less concentrated plasma.
|
|
|
|
The late distal tubule |
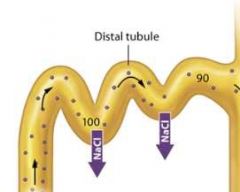
Once the filtrate enters the late distal tubule and collecting system, faculative water reabsorption may begin, so the concentration of the filtrate varies with the amount of water reabsorbed. If less water is reabsorbed, the concentration of the filtrate remains low as it passes through the late distal tubule and collecting system. The end result is the production of dilute urine, or urine with a concentration less than 300 mOsm. If, however, more water is reabsorbed, the concentration of the filtrate progressively increases as it passes through the late distal tubule and collecting system. The end result is the production of concentrated urine, or urine with a concentration greater than 300 mOsm. The upcoming sections examine the means by which both dilute urine and concentrated urine are produced. |
|
|
|
Production of Dilute urine |
The kidneys produce dilute urine when the solute concentration of the body's extracellular fluid is too low, which means the extracellular fluid contains excessive water. The filtrate entering the late distal tubule is already less concentrated than the surroudning interstitial fluid. For this reason the kidneys simply have to not reabsorb any additional water from the filtrate, or "turn off" faculative water reabsorption to produce dilute urine. This is accomplished through a reduction in ADH release, which renders the late distal tubule and collecting system essentially impermeable to water.
Note that the distal tubule and collecting system continue to reabsorb sodium and chloride ions from the filtrate, so the number of solutes in the filtrate decreases while the amount of water remains the same.This cayses the concentration of the filtrate to progressively decrease as it passes through the collecting system. When the amout of water in the extracellular fluid is greatly in excess, the osmolairty of plasma. Typically the volume of urine produced also increases when the urine is very dilute. As you learned, the normal volume of urine produced is about 1.8 liters per day. However this value can increase dramatically when ADH secretion is low. Note that urin volume is also influenced by many factors, including fluid intake genral health, diet, and blood pressure. It is also impacted by diuretics, which act on various parts of the renal tubule to block reabsorption of water or solutes and promote diuresis. |
|
|
|
Osmolarity Recap |
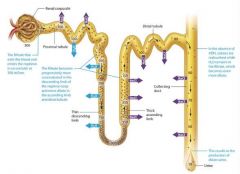
|
|
|
|
s |
s |
|
|
|
s |
s |
|
|
|
The Countercurrent Mechanism and the Production of Concentrated urine |
Oue kidneys are quite effective at conserving water and can produce urine with a concentration of about 1200 mOsm. Concentrated urine results from "turning on" faculative water reabsorption in the late distal tubule and collecting system by the release of ADH. Howver faculative water reabsorption requires more than simply the presence of ADH. Recall that water reabsorption happens only by osmosis, and osmosis being a passive process, will occur only if a concentration gradient is present to drive it. This means that faculative water reabsorption takes place only if the interstitial fluid surrouding the nepthon is more concentrated than the filtrate. The intersitital fluid within the renal cortex has about the same osmolarity as the intersitital fluid elsewhere in the body, approximately 300 mOsm. The filtrate entering the late distal tubule and cortical collecting duct has an osmolarity of about 100 mOsm, so it is less concentrated than the intersitital fluid. This creates a small but significant gradient to drive water reabsorption. However, by the time the filtrate enters the medullary collecting duct, its osmolarity has risen to a value about equal to that of interstital fluid and the gradient disappears. Therefore osmosis will not take place unles the nephrons work to create a concentration gradient within the renal medulla. This gradient known as the medullary osmotic gradient starts at 300 mOsm at the cortex/medulla border, and increases as we go deeper into the medulla. Within the deepest regions of the rneal medulla, it reaches a concentration of about 1200 mOsm, four times more concentrated than plasma. The medullary osmotic gradient is created and maintained by a system called the countercurrent mechanism. |
|
|
|
The counter current mechanism |
type of mechanism that involves the exchange of materials or heat between fluids flowing in opposite directions. In the kidneys, thismechansim consists of three factors:
1: a countercurrent multiplier system in the nephron loops of juxtamedullary nephrons
2: the recycling of urea in the medullary collecting ducts, and
3: a countercurrent exchanger in the vasa recta |
|
|
|
The Nephron Loop and the countercurrent multiplier |
Recall that there are two types of nephrons: cortical and juxtamedullary. Up to this point in the chapter we have been discussing the physiology of both types of nephron. Now however we shift our attention specifically to the physiology of juxtamedullary nephrons thos with long nephron loops that descend deeply into the renal medulla.
Within these long nephron loops we fins a system called the countercurrent multuplier, which helps to create the medullary osmotic gradient.
|
|
|
|
The countercurrent multiplier |
In this system, the term countercurrent refers to the fact that the filtrate in the two limbs of the nephron loop flows in opposite directions the filtrate in the descending limb flows toward the renal pelvis, and the filtrate in the ascending limb flows back up toward the renal cortex. Although the two limbs not directly touch, they are close enough to influence each other. The term multiplier means that the effect increases, or multiplies, as the process proceeds.
1: NaCl is actively transported from the filtrate in the thick ascending limb into the interstitial fluid, raising its NaCl concentration
2: The NaCl pumped into the interstitial fluid draws water out of the filatrate in the thin descending limb into the interstitial fluid by osmosis.
3: Due to the continuing loss of water, the NaCl concentration as it approaches the bottom of the loop.
4: The high NaCl concentration of the filtrate that reaches the thick ascending limb allows the NaCl reabsorption to continue.
All of these steps are occuring constantly; we seperataed them here for simplicity. You can see how the process work
The interstitial fluid is most concentrated in the deepest par of the renal medulla beacuase the amount of NaCl pumps out of the thick ascending limb is proportionanal to its concentration in the filtrate, and its concentration is highest at the baseof the loop. As the filtrate moves up the thick ascending limb and NaClis pumped out, the NaCl concentration in the filtrate decreases and less NaCl can be pumped into the interstitial fluid. |
|
|
|
NaCl is actively transported from the filtrate in the thick ascending limb into the interstitial fluid, raising its NaCl concentration |
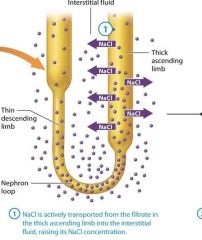
Na+/K+/2Cl- symporters pump NaCl from the cells of the thick ascending limb into the interstital fluid. This increases the interstitial fluids osmolarity |
|
|
|
The NaCl pumped into the interstitial fluid draws water out of the filtrate in the thin descending limb into the interstitial fluid by osmosis |
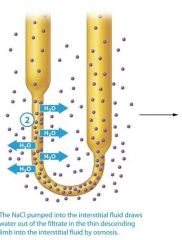
The concentrated interstitial fluid creates an osmotic gradient that draws water from the filtrate in the thin descending limb into the intersitial fluid. |
|
|
|
Due to the continuing loss of water, the NaCl concentration of the filtrate increasees as it approaches the bottom of the loop |
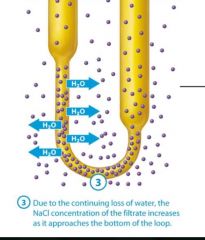
As water leaves the filtrate in the descending limb, NaCl remains, so the filtrate becomes progressively more concentrated as it reaches the bottom of the nephron loops. |
|
|
|
The high NaCl concentration of the filtrate that reaches the thick ascending limb allows the NaCl reabsorption to continue |
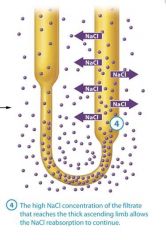
The filtrate reaches the thick ascending limb with a very high NaCl concentration This is a key because the symporters in the thick ascending limb will work only if the filtrate has a high NaCl concentration. The symporters then begin pumping NaCl into the interstitial fluid, and we return to step 1 |
|
|
|
The medullary Collecting sytem and Urea Recycling |
A second factor that helps to create the medullary osmotic gradient is the permeability of the medullary collecting system to urea. As water is reabsorbed from the filtrate, urea becomes more concentrated in the remaining fluid. In the medullary collecting ducts and papillary ducts, urea follows its concentration gradient and passively diffuses out of the filtrate and into the interstitial fluid, further concentrating the medullaryinterstitial fluid. Some urea then enters the thin descending limb,so it continuously recycles. Note that the urea diffusing out of the medullary collecting duct constitutes only a small amount of the total urea; much of the urea remains in the filtrate and ecreted in the urine. |
|
|
The vasa Recta and the counter current exchanger |
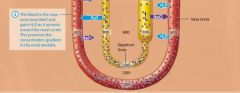
The steep medullary osmotic gradient created by the countercurrent multiplier and urea recycling is maintained by the vasa rects, the capillaries surrouding the nepthron loops of juxtamedulary nephrons. The vasa recta act as a special kind of vascular system called a countercurrent exchanger. Like the limbs of the nephron loop, the vasa recta descend into the renal medulla, and then following a hairpin turn, ascend toward the renal cortex. This arrangment of countercurrent flow, the blood flowing in the opposite direction from the filtrate, enables them to exchange susbstances. Lets take a closer look at how countercurrent exchange works, its illustrated in figure 24.22. First notice that the blood within the vasa recta has a concentration of about 300 mOsm as it enters the renal medulla. This means that as the descends into the medullo its is hypo-osmotic to the interstitial fluid. As we saw earlier, this situation causes water to leave the blood and enter the interstitial fluid by osmosis. In addition, more NaCl is present in the interstitial fluid by osmosis. In addition more NaCl is present in the interstitital fluid than in the blood, which causes NaCl to diffuse from the interstitial fluid into the blood. The blood in the vasa recta continues to pick up NaCl and lose water as it descends deeper into the renal medulla. By the time the blood reaches the deepest part of the medulla it has a concentration of about 1200 mOsm. However as the vasa recta ascend through the medulla the gradient is reversed, the blood is now hyperosmotic to the interstitial fluid, and the opposite process occures. NaCl now diffuses out of the blood and back into the interstitial fluid, and water moves by osmosis from the intersitital fluid into the blood. Notice what has happeneded here: All of the NaCl that was removed from the intersitital fluid by the blood in the descending limb of the vasa recta was exchanged or put back into the interstitial fluid by the blood in the ascending limb of the vasa rects. By the time the vasa recta exit the renal medulla, the blood has approximately the same concentration ( about 300 mOsm) it had upon entering the renal medulla. The return of the blood to its intitial osmolarity is critical, because it allows the vasa rects to deliver oygen and nutrietns to the cells of the medulla without depleting the medullary osmotic gradient necessary for water reabsorption and the production of concentrated urine. Numerous vasa recta and nephrons are actually in proximity to each other in the kidney. |
|
|
|
s |
s |
|
|
|
s |
s |
|
|
|
How the Countercurrent Mechansism Produces Concentrated Urine |
The countercurrent system is complicated so letst ake the time to summarize its function. First, keep in mind that the entire homeostatic function of this system is to conserve water for the body when needed. Water can be reabsorbed from collecting ducts only if ADH is present and a concentration gradient is there to drive osmosis. This medullary osmotic gradient doesnt exist on its own, so the kidneys must create and maintain it in the following ways:
The countercurrent multiplier of the thick ascending limb establishes the medullary interstitial gradient by pumping NaCl into the interstitial fluid.
Continued solute reabsorption, including urea recycling, from the filtrate in the medullary collecting duct adds to the gradient.
The countercurrent exchanger of the vasa recta allows perfusion of the inner medulla while maintaining the medullary interstitial gradient. |
|
|
|
Steps of concentrated urine production |
1: When the filtrate enters the cortical collecting duct in the renal medulla, there is no osmotic gradient between the filtrate and the interstitial fluid, so no water is reabsorbed.
2: In the presence of ADH, the concentrated medullary interstitial fluid creates a gradient for water reabsorption from the filtrate in the medullary collecting duct
3: Deeper into the medulla, intersitial fluid is more concentrated so water reabsorption continues form the medullary collecting duct.
4: Concentrated urine is produced. This process produces urine with a concentration up to 1200 mOsm. We cannot make more concentrated urine, because afterthis point there is no longer a gradient to drive osmosis. |
|
|
|
When the filtrate enters the cortical collecting duct in the renal medulla, there is no osmotic gradient between the filtrate and the interstitial fluid, so no water is reabsorbed. |
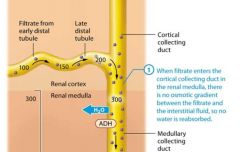
Filtrate entering the renal medulla has the same concentration as the interstitial fluid, so no gradient is present to drive water reabsorption. |
|
|
|
In the presence of ADH, the concentrated medullary interstitial fluid creates a gradient for water reabsorption from the filtrate in the medullary collecting duct |
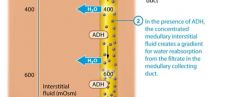
The interstitial fluid in the renal medulla is more concentrated than the filtrate. So as the filtrate passes deeper into the renal medulla through the medullary collecting duct, if ADH is present, water is drawn into the intersitital fluid by osmosis, thanks to the aquaporins and the medullary osmotic gradient. Water is reabsorbed until the filtrate and interstitial fluid are iso osmotic.
|
|
|
|
Deeper into the medulla, intersitial fluid is more concentrated so water reabsorption continues form the medullary collecting duct. |
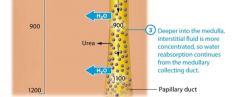
The process continues because the interstitial fluid becomes progresively more concentrated inthe deep renal medulla, allowing continued water reabsorption. |
|
|
|
Concentrated urine is produced. |
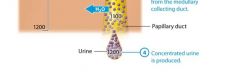
This process produces urine with a concentration up to 1200 mOsm. We cannot make more concentrated urine, because afterthis point there is no longer a gradient to drive osmosis. |
|
|
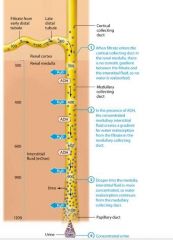
|
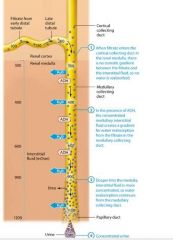
|
|
|
|
Urine Composition and Urinalysis |
Urine is the fluid that remains after tubular reabsorption and secretion have taken place. It normally contains water, sodium, potassium, chloride, and hydrogen ions; phosphates, sulfates; and metabolic waste products such as urea, creatinine, ammonia, and uric acid. It may also contain trace amounts of bicarbonate, calcium, and magnesium ions. |
|
|
|
Factors examined in Urinalysis |
Color Tanslucency Odor pH Specific Gravity |
|
|
|
Color |
Urine is colored by a yellow pigment called urochrome, a breakdown product of hemoglobin. The more concentrated the urine, the less water is presetn, so the same amount of urochrome makes the urine darker yellow. The color of urine may also be altered by certain foods, vitamins, drugs, and food dyes, or by the presence of blood. |
|
|
|
Translucency |
Regardless of color urine should always be translucent. Cloudy urine typically indicates an infection but may also indicate that the urine contains large quantities of protein. |
|
|
|
Odor |
Recently voided urine should have a mild odor. If urine is allowed to sit out, however, bacteria metabolize the urea in the urine to produce ammonia, giving it a stronger odor. The odor of urine may also be altered by certain disease states, such as diabetes mellitus or infection, or by eating certain foods, such as asparagus. |
|
|
|
pH |
The pH of urine is normally around 6.0 slightly acidic but it can range from 4.5 to 8.0. The reason for the minimum pH of 4.5 is that the H+ transport pumps in the distal tubule and collecting system cannot pump against a higher hydrogen ion gradient than this. |
|
|
|
Specific Gravity |
Specific gravity compares the amount of solutes in a solution to the amount in deionized water. Deionized water has no solutes and is assigned a specific gravity of 1.0; becuase urin has solutes, its specific gravity will be higher than that of water. This value typically ranges from 1.001 ( very dilute urine) to 1.035 (very concentrated urine) |
|
|
|
Renal Clearance |
Evaluation of renal function is very important in clinical settings to monitor the health of the kidneys. Although urinalysis may provide valuable clues about renal function, a more complete assessment is provided by measuring the rate at which the kidneys remove a substance from the blood, a process known as renal clearance. The renal clearance of the chemical is then used to estimate the GFR. Renal clearance and GFR both are measured in the same units: milliliters of plasma per minute. For a substance to provide an accurate measure of renal clearance and the GFR, the substance should be completely filitered and neither reabsorbed nor secreted. Substances secreted by renal tubules have renal clearance greater than their GFR, whereas those that are reabsorbed have renal clearance less than their GFR. To measure renal clearance, we therefore have a limited group of substances from to chooose. Two such commonly used substances are creatine and inulin. |
|
|
|
Creatinine |
creatinine is a waste product of the metabolism of muscle and other cells. Nearly all of the creatinine produced is excreted by the kidneys. When the kidneys are impired, the level of creatine in the blood tends to rise. Generally, a plasma creatine level above 1.2 mg/dl miligrams per deciliter is considered abnormal, but this varies with age, gender and body mass. Creatine excretion may be used to estimate the GFR by comparing the amount of creatine excreted in the urine to the plasma concentration of creatinine. The main difficulty with using creatinine as an indicator of glomerular filtation is that between 15% and 50% of creatine in the urine arrived there via secretion, not filtration. So a patient with very low GFR may still have a nearly normal result from this test. A more accurate assessment of the GFR can obtained using the substance inulin. Inulin is a complex carbohydrate found in plants such as garlic and artichokes that is filtered by the glomerulus, but is neither reabsorbed nor secreted by the renal tubule or collecting sytem. The GFR may be measured by injecting inulin and comparing its excretion in the urine to its plasma concentration. |
|
|
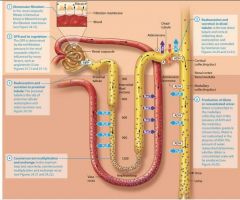
|
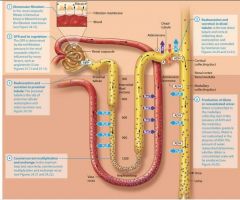
|
|
|
|
Anatomy of the Urinary Tract |
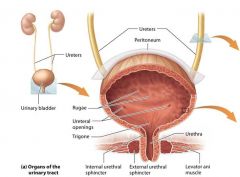
The urninary tract consists of the two ureters, the urinary bladder, and the urethra. |
|
|
|
Ureters |
The ureters transport urine from the kidneys to the urinary bladder. The ureters are generally about 25-30 cm long and 3-4 mm in diameter in and adult. They begin at roughly the level of the second lumbar vertebra, travel behind the peritoneum, and empty into the urninary bladder.
Like any hollow organ the ureters have a multilayered wall. The ureters deain posteriorly into the inferior urinary bladder. In this region a mechanism prevents urine from flowing backward through the ureter. As each ureter passes along the posterior urniary bladder, it travels obliquely through a "tunnel" in the bladder wall. As urine collects in the bladder, the pressure rises and compresses this tunnel, pinching the ureter closed and preventing backflow of urine.
|
|
|
|
Layers of the ureters from superficial to deep |
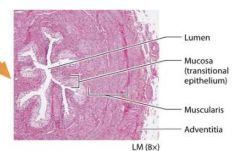
Adventitia
Muscularis
Mucosa |
|
|
|
Adventitia |
This superficial layer, known as the adventitia is fibrous connective tissue that supports the ureters. |
|
|
|
Muscularis |
The middle layer is the muscularis and its consists of smooth muscle. Like the smooth muscle of the alimentary canal, the smooth muscle in the muscularis contracts rhythemically via paristalsis to propel urine toward the urinary bladder. Waves of peristalsis course rhymically via peristalsis to propel urine toward the urinary bladder. Waves of peristalsis course through the muscularis as often as five time per minute, depending on the rate of urin production. |
|
|
|
Mucosa |
The innermost layer is the mucosa, a mucous membrane composed of transitional epithelium and its underlying basal lamina. As you leanred in chapter 4 transitional epitherlium is stratified with cells that can change from having a dome shape to being squamous. This property allows the epithelium to expand and recoil. |
|
|
|
Urinary Bladder |
The urinary bladder is a hollow distensible organ that sits on the floor of the pelvic cavity, suspended by a fold of parietal peritoneum. If collapses when empty but when distended, it becomes pear shaped and can hold up to about 700-800 ml of urine in males and sligtly less in females.
Like the ureters the wall of the urinary bladder has three tissue layers Notice that the floor of the urinary bladder contains a triangular area called the trigone (TRY-gohn; “triangle”). The trigone lacks rugae and appears smooth because its mucosa is tightly bound to the underlying muscularis. The two posterior corners of the trigone are formed by the two ureteral orifices (openings). These orifices have mucosal flaps that act as valves to prevent backflow of urine during elimination. The apex of the trigone is formed by the opening to the urethra, the internal urethral orifice. |
|
|
|
layers of the urinary blader from superficial to deep |
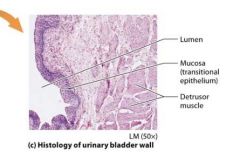
Adventitia
Detrusor Muscle
Mucosa |
|
|
|
Adventitia |
Adventitia. The adventitia, the most superficial layer, is composed of areolar connective tissue. On the superior surface of the urinary bladder is an additional serosa, which is a fold of the parietal peritoneum.
|
|
|
|
Detrusor muscle |
The middle tissue layer is composed of smooth muscle known as the detrusor muscle (dee-TROO-sor; “to push down”). The muscle fibers are arranged into inner longitudinal, middle circular, and outer longitudinal layers. The detrusor muscle forms a circular band around the opening of the urethra, called the internal urethral sphincter, shown in Figure 24.25a. |
|
|
|
Mucosa |
Mucosa. The mucosa is composed of transitional epithelium with an underlying basal lamina. It is a mucous membrane that produces mucus to protect the bladder epithelium from urine. When the bladder is not full, folds of mucosa called rugae (ROO-gee) are visible. What would happen if this mucus was not present? See A&P in the Real World: Interstitial Cystitis to find out.
|
|
|
|
Urethra |
The urethra is the terminal protion of the urniary tract; it drains urine from the urinary bladder to the outside of the body. Like the rest of the urnary tract, the urethra has an outer adventitia, middle muscularis, and inner mucose. It begins at the internal urethral orifice in the urinary bladder, which is surrounded by the internal urethral sphincter. This sphincter remains closed unless urin is being elminated. A second urethral sphincter the external urethral sphicncter, is formed from the levator ani muscle, the muscular floor of the pelvic cavity. This sphincter is composed of skeletal muscle and isunder voluntary control. Ther is no sphincter at the external orifice to the urethra in either males or females. |
|
|
|
Male Vs Female Urethras |
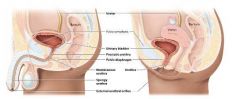
The urethra differs in males and females structurally as shown in figures 24.26 which means that functional differnces must exist as well. The female urethra is shorter about 4 cm in length and opend at the external urethral orifice between the vagina and the clitoris. It serves exclusively as a passage for urine. The male urethra is considerably longer about 20 cm in length and consists of three regions The location of the male urethra allows it to serve dual purpose: It transports both urine and semen. |
|
|
|
Three regions of the male urethra |
Prostatic Urethra
Membranous Urethra
Spongy Urethra |
|
|
|
Prostatic Urethra |
As the urethra exits the urniary bladder it pases through the prostate gland, which sits inferior to the urinary bladder. This section of the urethra is called the prostatic Urethra. |
|
|
|
Membranous Urethra |
The shortest segment known as the membranous urethra passes through the levator ani msucle |
|
|
|
Spongy urethra |
The longest segment of the male urethra the spongy uretha pases through the peis to the external urethral orifice. It is called the spongy urethra becuase it passes through an erectile body of the penis called the corpus spongiosium. |
|
|
|
Micturition |
Micturition also called urination or voiding is the discharge of urine form the urniary bladder to the outside of the body. It is activated by a reflex arc called the micturition reflex, which is mediated by the parasympathetic nervous sytem. This reflex arc is carried out by three components 1) Stretch receptors in the wall of the urinary bladder 2) sensory afferent nerve fibers that convey this information to the sacral protion of the spinal cord (S2 and S3) and (3) parasympatheric efferent fibers that travel to the detrusor muscle and internal sphincter of he urinary bladder |
|
|
|
Micturition |
When urine fills the bladder and stretches its walls, the micturition reflex occurs. In infants and youn children, this involuntary reflex is the primary mechanism by which the urinary bladder is emptied. |
|
|
|
micturition reflex arch |
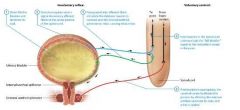
1: urine fills the bladder and stetches its wall
2: sttretch receptors send a signal via sensory afferent fibers to the sacral protion of the spinal cord
3: Parasympathetic efferent fibers stimulate the setrusor muscle to contract and the internal urethral sphincter to relax causing micturition. stages 4-5 occur after developement of the micturition center
4: Interneurons in the psinal cord communicate the "full bladder" signal to the micturition center in the pons.
5: If micturition is appropriate, the cerebral cortex facilitates this process by allowing the external urethral sphincter to relax, and urine is voided |
|
|
|
Development of Micturition |
As children mature, however pathways develop between these parasympathetic neurons and the brain that allow control over the external urethral sphincter. At this pont micturition is predominantly controlled by the micturition center in the pons, making the process voluntary. When the bladder is full two additional step then occure simultaneously with the involuntary process 4: Interneurons in the sinal cord communicate the full bladder signal to the micturition center in the pons 5: If micturiton is appropriate the cerebral cortex facilitates this process by allowing the external urethral sphincter to relax and urine is voided. Steps 4 and 5 dont occur until development of the micturition center. |
|
|
|
conscious control over micturition |
If micturition is not appropriate, then the detrusor muscle relaxes, the internal and external urethral sphincters remain closed, and the urge to urinate passes. The reflex generally initiates again within about an hour. This cycle repeats until the sensation of having to urinate becomes more acute. By the time about 500–600 ml has accumulated in the urinary bladder, the urge to urinate becomes too strong, voluntary control over the external urethral sphincter is lost, and micturition occurs. After micturition, the bladder contains only about 10 ml of urine. |
|

