![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
224 Cards in this Set
- Front
- Back
|
Causes of pre-hepatic jaundice?
|
Pre-hepatic
Pre-hepatic jaundice is caused by anything which causes an increased rate of hemolysis (breakdown of red blood cells). In tropical countries, malaria can cause jaundice in this manner. Certain genetic diseases, such as sickle cell anemia, spherocytosis, thalassemia and glucose 6-phosphate dehydrogenase deficiency can lead to increased red cell lysis and therefore hemolytic jaundice. Commonly, diseases of the kidney, such as hemolytic uremic syndrome, can also lead to coloration. Defects in bilirubin metabolism also present as jaundice, as in Gilbert's syndrome (a genetic disorder of bilirubin metabolism which can result in mild jaundice, which is found in about 5% of the population) and Crigler-Najjar syndrome. In jaundice secondary to hemolysis, the increased production of bilirubin, leads to the increased production of urine-urobilinogen. Bilirubin is not usually found in the urine because unconjugated bilirubin is not water-soluble, so, the combination of increased urine-urobilinogen with no bilirubin (since, unconjugated) in urine is suggestive of hemolytic jaundice. Laboratory findings include: Urine: no bilirubin present, urobilinogen > 2 units (i.e., hemolytic anemia causes increased heme metabolism; exception: infants where gut flora has not developed). Serum: increased unconjugated bilirubin. Kernicterus is associated with increased unconjugated bilirubin. |
|
|
Hepatocellular (hepatic) jaundice can be caused by?
|
Hepatocellular (hepatic) jaundice can be caused by acute or chronic hepatitis, hepatotoxicity, cirrhosis, drug induced hepatitis and alcoholic liver disease. Cell necrosis reduces the liver's ability to metabolize and excrete bilirubin leading to a buildup of unconjugated bilirubin in the blood. Other causes include primary biliary cirrhosis leading to an increase in plasma conjugated bilirubin because there is impairment of excretion of conjugated bilirubin into the bile. The blood contains abnormally raised amount of conjugated bilirubin and bile salts which are excreted in the urine. Jaundice seen in the newborn, known as neonatal jaundice, is common in newborns [6] as hepatic machinery for the conjugation and excretion of bilirubin does not fully mature until approximately two weeks of age. Rat fever (leptospirosis) can also cause hepatic jaundice. In hepatic jaundice, there is invariably cholestasis.
Laboratory findings depend on the cause of jaundice. Urine: Conjugated bilirubin present, urobilirubin > 2 units but variable (except in children). Kernicterus is a condition not associated with increased conjugated bilirubin. Plasma protein show characteristic changes. Plasma albumin level is low but plasma globulins are raised due to an increased formation of antibodies. Bilirubin transport across the hepatocyte may be impaired at any point between the uptake of unconjugated bilirubin into the cell and transport of conjugated bilirubin into biliary canaliculi. In addition, swelling of cells and oedema due to inflammation cause mechanical obstruction of intrahepatic biliary tree. Hence in hepatocellular jaundice, concentration of both unconjugated and conjugated bilirubin rises in the blood. In hepatocellular disease, there is usually interference in all major steps of bilirubin metabolism - uptake, conjugation and excretion. However, excretion is the rate-limiting step, and usually impaired to the greatest extent. As a result, conjugated hyperbilirubinaemia predominates |
|
|
Causes of post-hepatic jaundice?
|
Post-hepatic
Post-hepatic jaundice, also called obstructive jaundice, is caused by an interruption to the drainage of bile in the biliary system. The most common causes are gallstones in the common bile duct, and pancreatic cancer in the head of the pancreas. Also, a group of parasites known as "liver flukes" can live in the common bile duct, causing obstructive jaundice. Other causes include strictures of the common bile duct, biliary atresia, cholangiocarcinoma, pancreatitis and pancreatic pseudocysts. A rare cause of obstructive jaundice is Mirizzi's syndrome. In complete obstruction of the bile duct, no urobilinogen is found in the urine, since bilirubin has no access to the intestine and it is in the intestine that bilirubin gets converted to urobilinogen to be later released into the general circulation. In this case, presence of bilirubin (conjugated) in the urine without urine-urobilinogen suggests obstructive jaundice, either intra-hepatic or post-hepatic. The presence of pale stools and dark urine suggests an obstructive or post-hepatic cause as normal feces get their color from bile pigments. However, although pale stools and dark urine are a feature of biliary obstruction, they can occur in many intra-hepatic illnesses and are therefore not a reliable clinical feature to distinguish obstruction from hepatic causes of jaundice.[8] Patients also can present with elevated serum cholesterol, and often complain of severe itching or "pruritus" because of the deposition of bile salts. No single test can differentiate between various classifications of jaundice. A combination of liver function tests is essential to arrive at a diagnosis. |
|
|
Albumin (Alb)
Reference range 3.5 to 5.3 g/dL Albumin decrease caused by? |
Albumin (Alb)
Reference range 3.5 to 5.3 g/dL Albumin is a protein made specifically by the liver, and can be measured cheaply and easily. It is the main constituent of total protein; the remaining froglobulins). Albumin levels are decreased in chronic liver disease, such as cirrhosis. It is also decreased in nephrotic syndrome, where it is lost through the urine |
|
|
Alanine transaminase (ALT)
Reference range 7 to 56 IU/L[4] Alanine transaminase (ALT) rise due to? |
Alanine transaminase (ALT)
Reference range 7 to 56 IU/L[4] Alanine transaminase (ALT), also called serum glutamic pyruvate transaminase (SGPT) or alanine aminotransferase (ALAT) is an enzyme present in hepatocytes (liver cells). When a cell is damaged, it leaks this enzyme into the blood, where it is measured. ALT rises dramatically in acute liver damage, such as viral hepatitis or paracetamol (acetaminophen) overdose. Elevations are often measured in multiples of the upper limit of normal (ULN). |
|
|
Aspartate transaminase (AST)
Reference range 5 to 40 IU/L[4] Aspartate transaminase (AST) rise due to? |
Aspartate transaminase (AST)
Reference range 5 to 40 IU/L[4] Aspartate transaminase (AST) also called serum glutamic oxaloacetic transaminase (SGOT) or aspartate aminotransferase (ASAT) is similar to ALT in that it is another enzyme associated with liver parenchymal cells. It is raised in acute liver damage, but is also present in red blood cells, and cardiac and skeletal muscle and is therefore not specific to the liver. The ratio of AST to ALT is sometimes useful in differentiating between causes of liver damage.[5][6] Elevated AST levels are not specific for liver damage, and AST has also been used as a cardiac marker. |
|
|
Alkaline phosphatase (ALP)
Reference range 30 to 120 IU/L[4] Alkaline phosphatase (ALP) rise due to? |
Alkaline phosphatase (ALP)
Reference range 30 to 120 IU/L[4] Alkaline phosphatase (ALP) is an enzyme in the cells lining the biliary ducts of the liver. ALP levels in plasma will rise with large bile duct obstruction, intrahepatic cholestasis or infiltrative diseases of the liver. ALP is also present in bone and placental tissue, so it is higher in growing children (as their bones are being remodelled) and elderly patients with Paget's disease. |
|
|
Total bilirubin (TBIL)
Reference range 0.2–1.2 mg/dL Bilirubin metabolism basic? |
Total bilirubin (TBIL)
Reference range 0.2–1.2 mg/dL Bilirubin is a breakdown product of heme (a part of hemoglobin in red blood cells). The liver is responsible for clearing the blood of bilirubin. It does this by the following mechanism: Bilirubin is taken up into hepatocytes, conjugated (modified to make it water-soluble), and secreted into the bile, which is excreted into the intestine. Increased total bilirubin causes jaundice, and can signal a number of problems: 1. Prehepatic: Increased bilirubin production. This can be due to a number of causes, including hemolytic anemias and internal hemorrhage. 2. Hepatic: Problems with the liver, which are reflected as deficiencies in bilirubin metabolism (e.g., reduced hepatocyte uptake, impaired conjugation of bilirubin, and reduced hepatocyte secretion of bilirubin). Some examples would be cirrhosis and viral hepatitis. 3. Posthepatic: Obstruction of the bile ducts, reflected as deficiencies in bilirubin excretion. (Obstruction can be located either within the liver or in the bile duct). [edit] Direct bilirubin (conjugated bilirubin) Reference range 0.1–0.4 mg/dL The diagnosis is narrowed down further by looking at the levels of direct bilirubin. If direct (i.e. conjugated) bilirubin is normal, then the problem is an excess of unconjugated bilirubin, and the location of the problem is upstream of bilirubin excretion. Hemolysis, viral hepatitis, or cirrhosis can be suspected. If direct bilirubin is elevated, then the liver is conjugating bilirubin normally, but is not able to excrete it. Bile duct obstruction by gallstones or cancer should be suspected. |
|
|
Gamma glutamyl transpeptidase (GGT) raised in?
Reference range 0 to 42 IU/L[4] |
Gamma glutamyl transpeptidase (GGT)
Reference range 0 to 42 IU/L[4] Although reasonably specific to the liver and a more sensitive marker for cholestatic damage than ALP, Gamma glutamyl transpeptidase (GGT) may be elevated with even minor, sub-clinical levels of liver dysfunction. It can also be helpful in identifying the cause of an isolated elevation in ALP (GGT is raised in chronic alcohol toxicity). |
|
|
Elevated serum GGT activity can be found in ?
|
Elevated serum GGT activity can be found in diseases of the liver, biliary system, and pancreas. In this respect, it is similar to alkaline phosphatase (ALP) in detecting disease of the biliary tract. Indeed, the two markers correlate well, though there is conflicting data about whether GGT has better sensitivity.[9][10] In general, ALP is still the first test for biliary disease. The main value of GGT over ALP is in verifying that ALP elevations are, in fact, due to biliary disease; ALP can also be increased in certain bone diseases, but GGT is not.[10] More recently it has also been found to be elevated in persons with cardiovascular diseases and is under active investigation as a cardiovascular risk marker.
GGT is elevated by large quantities of alcohol ingestion.[11] Isolated elevation or disproportionate elevation compared to other liver enzymes (such as ALP or ALT) may indicate alcohol abuse or alcoholic liver disease.[12] It may indicate excess alcohol consumption up to 3 or 4 weeks prior to the test. The mechanism for this elevation is unclear. Alcohol may increase GGT production by inducing hepatic microsomal production, or it may cause the leakage of GGT from hepatocytes.[13] Numerous drugs can raise GGT levels, including barbiturates and phenytoin.[14] Others include NSAIDs, St. John's wort, and aspirin.[citation needed] Elevated levels of GGT may also be due to congestive heart failure |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Purine analogs mechanism?
|
Purine analogs
These are relatives of the purines used in DNA synthesis. Because they interfere with DNA synthesis, they interfere with the rapid cell proliferation needed for immune responses. Azathioprine (trade name = Imuran) is a widely-used purine analog. Unfortunately, these drugs also interfere with the many other tissues that depend on rapid cell division (e.g., lining of the intestine, hair follicles) so they have many unpleasant side effects. Therefore, the search for agents that specifically target immune cells goes on. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Corticosteroids mechanism?
|
Corticosteroids
These relatives of cortisol interfere with a transcription factor needed to turn on the genes for T cells to become activated. Prednisone and prednisolone are most commonly used. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Tacrolimus (Prograf®) and cyclosporine (Neoral®) mechanism?
|
Tacrolimus (Prograf®) and cyclosporine (Neoral®)
These are natural products isolated from microbial cultures. They inhibit the signaling pathway used by T cells to turn on their genes for activation, e.g., for IL-2 secretion. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Rapamycin mechanism?
|
Rapamycin
This is a macrolide antibiotic produced by an actinomycete found on Easter Island (which the inhabitants call Rapa Nui — hence the name). Rapamycin inhibits T cell proliferation, and shows great promise in reducing the problems of transplant rejection. Rapamycin is also known as sirolimus and is sold under the trade name Rapamune. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Mycophenolate mofetil mechanism?
|
Mycophenolate mofetil
This small molecule inhibits an enzyme needed by B and T cells for purine synthesis. Other types of cells are not dependent on the enzyme so side effects are mild. The trade name is CellCept. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Antithymocyte globulin (ATG) mechanism?
|
Antithymocyte globulin (ATG)
This preparation contain antibodies — raised in horses or rabbits — directed against T cells. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Monoclonal antibodies
Several preparations are now used: •Muromonab-CD3 (OKT3) mechanism? |
Monoclonal antibodies
Several preparations are now used: •Muromonab-CD3 (OKT3) and two humanized anti-CD3 monoclonals. They bind to the CD3 molecule on the surface of T cells. •Daclizumab and basiliximab. Target the IL-2 receptor and thus inhibit only activated T cells. •Alemtuzumab (Campath-1H®). Binds to CD52, a molecule found on lymphocytes and depletes both T cells and B cells. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Monoclonal antibodies
Several preparations are now used: •Daclizumab and basiliximab mechanism? |
Monoclonal antibodies
Several preparations are now used: •Muromonab-CD3 (OKT3) and two humanized anti-CD3 monoclonals. They bind to the CD3 molecule on the surface of T cells. •Daclizumab and basiliximab. Target the IL-2 receptor and thus inhibit only activated T cells. •Alemtuzumab (Campath-1H®). Binds to CD52, a molecule found on lymphocytes and depletes both T cells and B cells. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Monoclonal antibodies
Several preparations are now used: •Alemtuzumab (Campath-1H®) mechanism? |
Monoclonal antibodies
Several preparations are now used: •Muromonab-CD3 (OKT3) and two humanized anti-CD3 monoclonals. They bind to the CD3 molecule on the surface of T cells. •Daclizumab and basiliximab. Target the IL-2 receptor and thus inhibit only activated T cells. •Alemtuzumab (Campath-1H®). Binds to CD52, a molecule found on lymphocytes and depletes both T cells and B cells. |
|
|
Immunosuppression is the treatment of the patient with agents that inhibit the immune response. Belatacept mechanism?
|
Belatacept
This is a protein, produced by recombinant DNA technology, that combines •the extracellular portion of CTLA-4 ("cytotoxic T-lymphocyte-associated antigen 4", one of the ligands for B7) with •the Fc region (the C-terminal two-thirds of the constant region) [View] of a human IgG1 antibody. It blocks the "Signal Two" needed to activate T cells |
|
|
Digoxin mechanism of action?
|
Digoxin binds to a site on the extracellular aspect of the α-subunit of the Na+/K+ ATPase pump in the membranes of heart cells (myocytes) and decreases its function. This causes an increase in the level of sodium ions in the myocytes, which leads to a rise in the level of intracellular calcium ions. This occurs because the sodium/calcium exchanger on the plasma membrane depends on a constant inward sodium gradient to pump out calcium. Digoxin decreases sodium concentration gradient and the subsequent calcium outflow, thus raising the calcium concentration in myocardiocytes and pacemaker cells.
Increased intracellular calcium lengthens Phase 4 and Phase 0 of the cardiac action potential, which leads to a decrease in heart rate.[16] Increased amounts of Ca2+ also leads to increased storage of calcium in the sarcoplasmic reticulum, causing a corresponding increase in the release of calcium during each action potential. This leads to increased contractility, the force of contraction, of the heart. There is also evidence that digoxin increases vagal activity, thereby decreasing heart rate by slowing depolarization of pacemaker cells in the AV node.[17] This negative chronotropic effect would therefore be synergistic with the direct effect on cardiac pacemaker cells. Digoxin is used widely in the treatment of various arrhythmias. |
|
|
scapular rotators ?
|
scapular rotators - trapezius, serratus anterior, rhomboids and levator scapulae
|
|
|
The rotator cuff is composed of four muscles?
|
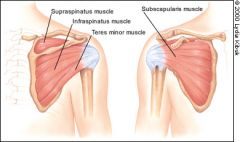
The rotator cuff is composed of four muscles: the supraspinatus, infraspinatus, teres minor and subscapularis
|
|
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles do what?
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles support posture; the trapezius and the serratus anterior muscles help rotate the scapula upward, and the trapezius and the rhomboids aid scapular retraction.
|
|
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles support posture; the trapezius and the serratus anterior muscles help do what?
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles support posture; the trapezius and the serratus anterior muscles help rotate the scapula upward, and the trapezius and the rhomboids aid scapular retraction.
|
|
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles support posture; the trapezius and the serratus anterior muscles help rotate the scapula upward, and the trapezius and the rhomboids aid in what in relation to the scapular?
|
Scapular stability collectively involves the trapezius, serratus anterior and rhomboid muscles. The levator scapular and upper trapezius muscles support posture; the trapezius and the serratus anterior muscles help rotate the scapula upward, and the trapezius and the rhomboids aid scapular retraction.
|
|
|
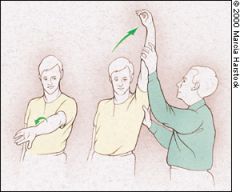
NEER'S TEST
Neer's impingement sign is elicited when the patient's rotator cuff tendons are pinched under the coracoacromial arch. The test4 is performed by placing the arm in forced flexion with the arm fully pronated (Figure 5). The scapula should be stabilized during the maneuver to prevent scapulothoracic motion. Pain with this maneuver is a sign of subacromial impingement. |
NEER'S TEST
Neer's impingement sign is elicited when the patient's rotator cuff tendons are pinched under the coracoacromial arch. The test4 is performed by placing the arm in forced flexion with the arm fully pronated (Figure 5). The scapula should be stabilized during the maneuver to prevent scapulothoracic motion. Pain with this maneuver is a sign of subacromial impingement. |
|
|
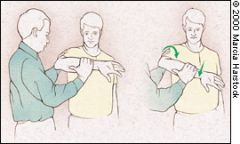
HAWKINS' TEST
The Hawkins' test is another commonly performed assessment of impingement.5 It is performed by elevating the patient's arm forward to 90 degrees while forcibly internally rotating the shoulder (Figure 6). Pain with this maneuver suggests subacromial impingement or rotator cuff tendonitis. One study6 found Hawkins' test more sensitive for impingement than Neer's test. |
HAWKINS' TEST
The Hawkins' test is another commonly performed assessment of impingement.5 It is performed by elevating the patient's arm forward to 90 degrees while forcibly internally rotating the shoulder (Figure 6). Pain with this maneuver suggests subacromial impingement or rotator cuff tendonitis. One study6 found Hawkins' test more sensitive for impingement than Neer's test. |
|
|
CROSS-ARM TEST
|
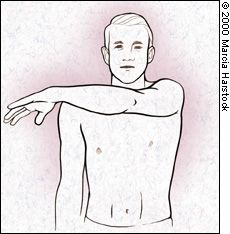
CROSS-ARM TEST
Patients with acromioclavicular joint dysfunction often have shoulder pain that is mistaken for impingement syndrome. The cross-arm test isolates the acromioclavicular joint. The patient raises the affected arm to 90 degrees. Active adduction of the arm forces the acromion into the distal end of the clavicle (Figure 7). Pain in the area of the acromioclavicular joint suggests a disorder in this region. |
|
|
APPREHENSION TEST
The anterior apprehension test is performed with the patient supine or seated and the shoulder in a neutral position at 90 degrees of abduction. The examiner applies slight anterior pressure to the humerus (too much force can dislocate the humerus) and externally rotates the arm (Figure 8). Pain or apprehension about the feeling of impending subluxation or dislocation indicates anterior glenohumeral instability. |
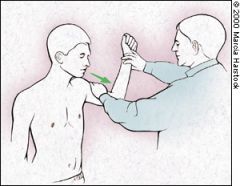
APPREHENSION TEST
The anterior apprehension test is performed with the patient supine or seated and the shoulder in a neutral position at 90 degrees of abduction. The examiner applies slight anterior pressure to the humerus (too much force can dislocate the humerus) and externally rotates the arm (Figure 8). Pain or apprehension about the feeling of impending subluxation or dislocation indicates anterior glenohumeral instability. |
|
|
YERGASON TEST
Patients with rotator cuff tendonitis frequently have concomitant inflammation of the biceps tendon. The Yergason test is used to evaluate the biceps tendon.9 In this test, the patient's elbow is flexed to 90 degrees with the thumb up. The examiner grasps the wrist, resisting attempts by the patient to actively supinate the arm and flex the elbow (Figure 9). Pain with this maneuver indicates biceps tendonitis. |
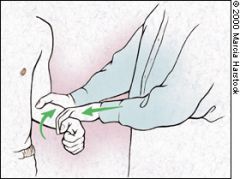
YERGASON TEST
Patients with rotator cuff tendonitis frequently have concomitant inflammation of the biceps tendon. The Yergason test is used to evaluate the biceps tendon.9 In this test, the patient's elbow is flexed to 90 degrees with the thumb up. The examiner grasps the wrist, resisting attempts by the patient to actively supinate the arm and flex the elbow (Figure 9). Pain with this maneuver indicates biceps tendonitis. |
|
|
SULCUS SIGN
With the patient's arm in a neutral position, the examiner pulls downward on the elbow or wrist while observing the shoulder area for a sulcus or depression lateral or inferior to the acromion. The presence of a depression indicates inferior translation of the humerus and suggests inferior glenohumeral instability (Figure 10). The examiner should remember that many asymptomatic patients, especially adolescents, normally have some degree of instability.10 |
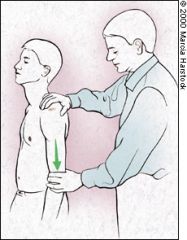
SULCUS SIGN
With the patient's arm in a neutral position, the examiner pulls downward on the elbow or wrist while observing the shoulder area for a sulcus or depression lateral or inferior to the acromion. The presence of a depression indicates inferior translation of the humerus and suggests inferior glenohumeral instability (Figure 10). The examiner should remember that many asymptomatic patients, especially adolescents, normally have some degree of instability.10 |
|
|
SPURLING'S TEST
In a patient with neck pain or pain that radiates below the elbow, a useful maneuver to further evaluate the cervical spine is Spurling's test. The patient's cervical spine is placed in extension and the head rotated toward the affected shoulder. An axial load is then placed on the spine (Figure 11). Reproduction of the patient's shoulder or arm pain indicates possible cervical nerve root compression and warrants further evaluation of the bony and soft tissue structures of the cervical spine. |
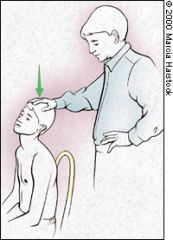
SPURLING'S TEST
In a patient with neck pain or pain that radiates below the elbow, a useful maneuver to further evaluate the cervical spine is Spurling's test. The patient's cervical spine is placed in extension and the head rotated toward the affected shoulder. An axial load is then placed on the spine (Figure 11). Reproduction of the patient's shoulder or arm pain indicates possible cervical nerve root compression and warrants further evaluation of the bony and soft tissue structures of the cervical spine. Cervical Disc Disease No physical examination in a patient with shoulder pain is complete without excluding cervical spine disease. Referred or radicular pain from disc disease should be considered in patients who have shoulder pain that does not respond to conservative treatment. The patient should be questioned about neck pain and previous neck injury, and the examiner should note whether pain worsens with turning of the neck, which suggests disc disease. Pain that originates from the neck or radiates past the elbow is often associated with a neck disorder. Plain film is a useful screening tool for degenerative cervical disc disease. Further work-up and imaging studies depend on the differential diagnosis and the treatment plan. |
|
|
◦The most common epithelial malignancies are ? (5)
|
◦The most common epithelial malignancies are lung (15%), pancreas (13%), colon/rectum (6%), kidney (5%) and breast (4%).
|
|
|
Initial investigations for malignancy should include?
|
•Initial investigations should include FBC (iron deficiency may indicate an occult gastrointestinal malignancy), renal function tests and electrolytes, LFTs, calcium, lactate dehydrogenase and urinalysis (microscopic haematuria may indicate genitourinary malignancy).
•CXR. •Myeloma screen (when there are isolated or multiple lytic bone lesions). •Symptom-directed endoscopy. •Computed tomography (CT) scan of the chest, abdomen and pelvis. •Prostate-specific antigen (PSA) in men. •Cancer antigen 125 (CA-125) in women with peritoneal malignancy or ascites. •Alpha-fetoprotein (AFP) and human chorionic gonadotrophin (hCG) (particularly in the presence of midline nodal disease). •Testicular ultrasound in men with presentations compatible with germ-cell tumours. •Biopsy and standard histological examination, with immunohistochemistry where necessary, to distinguish carcinoma from other malignant diagnoses. |
|
|
B symptoms ? (3)
|
B symptoms refer to systemic symptoms of fever, night sweats, and weight loss which can be associated with both Hodgkin's lymphoma and non-Hodgkin's lymphoma. The presence or absence of B symptoms has prognostic significance and is reflected in the staging of these lymphomas.
|
|
|
•Tumour markers should only be measured as follows:◦AFP and hCG in presentations compatible with ?
|
•Tumour markers should only be measured as follows:◦AFP and hCG in presentations compatible with germ-cell tumours (particularly mediastinal and/or retroperitoneal masses and in young men).
◦AFP in presentations compatible with hepatocellular cancer. ◦PSA in presentations compatible with prostate cancer. ◦CA-125 in presentations compatible with ovarian cancer (including inguinal node, chest, pleural, peritoneal or retroperitoneal presentations). Carefully interpret the results because of limited test specificity. |
|
|
•Tumour markers should only be measured as follows: ◦AFP in presentations compatible with ?
|
•Tumour markers should only be measured as follows:◦AFP and hCG in presentations compatible with germ-cell tumours (particularly mediastinal and/or retroperitoneal masses and in young men).
◦AFP in presentations compatible with hepatocellular cancer. ◦PSA in presentations compatible with prostate cancer. ◦CA-125 in presentations compatible with ovarian cancer (including inguinal node, chest, pleural, peritoneal or retroperitoneal presentations). Carefully interpret the results because of limited test specificity. |
|
|
•Tumour markers should only be measured as follows: ◦CA-125 in presentations compatible with ?
|
•Tumour markers should only be measured as follows:◦AFP and hCG in presentations compatible with germ-cell tumours (particularly mediastinal and/or retroperitoneal masses and in young men).
◦AFP in presentations compatible with hepatocellular cancer. ◦PSA in presentations compatible with prostate cancer. ◦CA-125 in presentations compatible with ovarian cancer (including inguinal node, chest, pleural, peritoneal or retroperitoneal presentations). Carefully interpret the results because of limited test specificity. |
|
|
Cor pulmonale is defined as?
|
Cor pulmonale is defined as an alteration in the structure and function of the right ventricle caused by a primary disorder of the respiratory system. Pulmonary hypertension is the common link between lung dysfunction and the heart in cor pulmonale. Right-sided ventricular disease caused by a primary abnormality of the left side of the heart or congenital heart disease is not considered cor pulmonale, but cor pulmonale can develop secondary to a wide variety of cardiopulmonary disease processes. Although cor pulmonale commonly has a chronic and slowly progressive course, acute onset or worsening cor pulmonale with life-threatening complications can occur
|
|
|
For cor pulmonale to come about, mean pulmonary arterial pressure is usually ?mmHg.
|
The structure and function of the right ventricle is adversely affected by pulmonary arterial hypertension, induced by a disease process affecting the lungs, their ventilation or blood supply. For cor pulmonale to come about, mean pulmonary arterial pressure is usually >20 mmHg. Complete right ventricular failure usually ensues if mean pulmonary arterial pressure is ≥40 mmHg.
It is thought that chronic hypoxia leads to pulmonary arteriolar constriction through excessive action of the physiological mechanism that acts to maintain the balance of ventilation and perfusion in the lungs. Other mechanisms that may raise mean pulmonary arterial pressure in cases of cor pulmonale include: •Chronic hypercapnoea and respiratory acidosis causing pulmonary vasoconstriction. •Anatomic disruption of the pulmonary vascular bed due to primary lung disease (for example in emphysema, pulmonary thromboembolic disease and pulmonary fibrosis). •Increased blood viscosity due to lung disease and its effects (for example in secondary polycythaemia). A wide range of pulmonary and cardiopulmonary disease processes may cause the condition. It is usually a chronic and progressive process, but does occur acutely due to sudden causes of pulmonary hypertension, usually following pulmonary embolism. If right-heart failure occurs due to primary disease of the left side of the heart, or because of a congenital cardiac lesion, then it is not normally considered to be cor pulmonale. |
|
|
Common symptoms that may suggest the presence of cor pulmonale ?
|
Common symptoms that may suggest the presence of cor pulmonale in a patient with pulmonary or cardiopulmonary disease include:
•Worsening tachypnoea (particularly at rest) •Fatigue and lassitude •Ankle swelling •Worsening exertional dyspnoea (with deterioration in exercise tolerance) •Worsening cough (particularly if non-productive) •Angina-type chest discomfort – often non-responsive to nitrates (thought to be due to right ventricular ischaemia or stretching of pulmonary artery during exertion) •Haemoptysis (due to pulmonary arteriolar rupture or leakage) •Hoarseness – occurs occasionally (due to compression of the left recurrent laryngeal nerve by dilated pulmonary artery) •Exertional syncope – a late symptom (indicating severe disease) •Late-stage hepatic congestion can cause symptoms (anorexia, jaundice and right-upper-quadrant abdominal discomfort) |
|
|
Signs of cor pulmonale?
|
Signs
•Cyanosis and plethora •Chest markedly hyper-expanded •Laboured respiratory effort •Intercostal recession •Decreased air entry, crackles and wheeze in chest due to underlying pulmonary pathology •Systolic bruits over lung fields – due to turbulent hyperdynamic pulmonary artery flow •Left parasternal or subxiphoid heave (sign of right ventricular hypertrophy) •Distended neck veins with raised and/or prominent JVP and visible a or v waves •3rd/4th heart sounds and pan-systolic murmur of tricuspid regurgitation over right heart •Split second heart sound with loud pulmonary component •Systolic ejection murmur with sharp ejection click over pulmonary artery (advanced sign) •Diastolic pulmonary regurgitation murmur over pulmonary artery (advanced sign) •Marked hepatojugular reflux due to hepatic congestion •Hepatomegaly ± liver pulsatility if significant associated tricuspid regurgitation •Jaundice in advanced cases •Ascites in advanced cases •Peripheral pitting oedema |
|
|
Causative diseases for cor pulmonale?
|
Causative diseases
Those due to secondary pulmonary arterial compromise •Chronic obstructive pulmonary disease (by far the commonest) •Other causes of parenchymal lung disease, e.g. idiopathic fibrosing alveolitis, emphysema, pneumoconiosis, cystic fibrosis •Neuromuscular disorders causing chronic hypoventilation, e.g. polio, myasthenia gravis, motor neurone disease •Obstructive or central sleep apnoea/Pickwickian syndrome (obesity hypoventilation syndrome)5 •Thoracic deformity, e.g. kyphoscoliosis •Alveolar capillary dysplasia •Neonatal pulmonary disease and its sequelae, e.g. bronchopulmonary dysplasia Those due to primary disease of the pulmonary arterial vessels •Recurrent pulmonary emboli •Other pulmonary veno-occlusive disease •Pulmonary vasculitis •Sickle cell disease •Altitude sickness/pulmonary vasoconstriction due to chronic altitude exposure •Primary pulmonary hypertension |
|
|
Management of cor pulmonale?
|
Management
Acute cor pulmonale is treated by trying to rapidly correct the underlying precipitant which is often acute pulmonary embolism or an infective exacerbation of COPD. Standard treatment for these conditions is used in an attempt to correct the underlying cause of acute right heart failure. Similarly, in chronic cor pulmonale, treatment of the underlying cause is combined with specific management as below: •Long-term oxygen therapy (LTOT)/Nocturnal Oxygen Therapy (NOT) have been shown to improve quality of life and survival in patients with severe chronic hypoxia due to lung disease, by reducing pulmonary arteriolar constriction and improving/slowing the progression of cor pulmonale.6 They are usually recommended where PaO2 is <55 mmHg or SaO2 is <88%. A Cochrane review has confirmed these benefits but shown a lack of efficacy for patients with only mild to moderate hypoxaemia/patients that only desaturate at night.7 Where there is clear clinical/investigational evidence of cor pulmonale, and higher mental/cognitive impairment attributable to hypoxia complicating chronic lung disease, LTOT/NOT may be given with oxygenation above these values.2 Great care must be taken to ensure the safety of patients who continue to smoke, as oxygen is highly combustible, and many clinicians will not give oxygen therapy to smokers (another reason being negation of its benefit by the presence of elevated carboxyhaemoglobin levels in smokers). •Diuretics such as furosemide and bumetanide are frequently utilised, particularly where the right ventricular filling volume is markedly elevated, and in the management of associated peripheral oedema. Care must be taken to avoid over-diuresis which can impair the function of both ventricles. It may also induce a hypokalaemic metabolic alkalosis which can lessen respiratory drive through reducing the hypercapnoeic stimulus to breathe. Intravenous diuretics may be needed in patients with acute decompensation and severe peripheral oedema, due to poor absorption of oral medication from the oedematous gut. •Vasodilators such as nifedipine and diltiazem have been shown to have modest physiological effects though there is no convincing trial evidence of their efficacy. •Inotropic drugs, particularly digoxin, are frequently used but there is little evidence for their efficacy in right heart failure, in contrast to their use with left ventricular failure. •Methylxanthine bronchodilators such as theophylline are frequently used for their beneficial effect on bronchial tone and concomitant mild positive inotropic effect.2 •Anticoagulation is used where patients have venous thromboembolism as the underlying cause of their cor pulmonale, and where there are significant risk factors for venous thromboembolic disease in patients with chronic lung disease and cor pulmonale. There is little evidence of tangible benefit in terms of survival in cases due to secondary pulmonary hypertension, in contrast to their proven benefit in primary pulmonary hypertension.2 •Venesection is used with caution in some patients who have severe secondary polycythaemia (usually defined as haematocrit >0.65) due to chronic hypoxia. It has been shown to improve symptomatology, but there is no evidence of improved survival.2 •Transplantation of single/double lung or heart/lung is used in some extreme cases of cor pulmonale and significantly improves outlook. The underlying cause must usually be unrelated to smoking to reduce the likelihood of other pathology that would give poorer outcomes. |
|
|
Complications of cor pulmonale?
|
Complications
•Exertional syncope •Hypoxia and significantly limited exercise tolerance •Peripheral oedema •Peripheral venous insufficiency •Tricuspid regurgitation •Hepatic congestion and cardiac cirrhosis •Death Prognosis This is dependent on the nature of the underlying cause and its rate of progression. The 2-year mortality for cor pulmonale complicating COPD is relatively high, particularly for those who continue to smoke.2 Overall 5-year mortality is around 60%, even in treated patients. Prognosis appears to be significantly improved by smoking cessation and correct use of LTOT/NOT. Long-term oxygen therapy (LTOT)/Nocturnal Oxygen Therapy (NOT) |
|
|
If you are the attending doctor during the last illness of a person who dies, you have a statutory duty[8] to issue ?
|
If you are the attending doctor during the last illness of a person who dies, you have a statutory duty[8] to issue a medical certificate of the cause of death (death certificate). Conversely, if you did not attend the deceased during his or her last illness you must not complete the death certificate.
You must state the cause(s) of death on the certificate to the best of your knowledge and belief. You have a duty to deliver the death certificate to the registrar of births and deaths: in practice, the certificate is often given to a relative of the deceased, then handed to the registrar by the relative (or other informant) who visits the register office to have the death registered. |
|
|
A death should be referred to the coroner if?
|
A death should be referred to the coroner if:
• the cause of death is unknown • the deceased was not seen by the certifying doctor either after death or within the 14 days before death • the death was violent or unnatural or suspicious • the death may be due to an accident (whenever it occurred) • the death may be due to self-neglect or neglect by others • the death may be due to an industrial disease or related to the deceased's employment • the death may be due to an abortion • the death occurred during an operation or before recovery from the effects of an anaesthetic • the death may be a suicide • the death occurred during or shortly after detention in police or prison custody |
|
|
Unexpected (“sudden”) deaths - duties of a doctor to attend?
|
Unexpected (“sudden”) deaths
If death occurs in the patient’s home, or in a residential or nursing home, we recommend a visit by the GP with whom the patient was registered, to examine the body and confirm death, although this is not a statutory requirement. Unlike for expected deaths, in the event of an unexpected death out-of-hours it would be helpful if an OOH GP does attend, therefore helping to prevent the potentially unnecessary attendance of the emergency services. The GP should then report the death to the coroner (usually through the local police). In any other circumstances, the request to attend is likely to have come from the police or ambulance service. It is usually wise, and especially in the case of an on-call doctor, to decline to attend and advise that the services of a Forensic Medical Examiner police surgeon be obtained by the caller. |
|
|
The law requires a doctor to notify the cause of death of any patient whom he/she has attended during that patient’s last illness to ?
|
The law requires a doctor to notify the cause of death of any patient whom he/she has attended during that patient’s last illness to the Registrar of Births and Deaths. The doctor is required to notify the cause of death as a certificate, on a form prescribed, stating to the best of his/her knowledge and belief, the cause of death. It should be noted that the strict interpretation of the law is that the doctor shall notify the cause of death, not the fact. Thus, a doctor does not certify that death has occurred, only what in his/her opinion was the cause, assuming that death has taken place. Arising out of this interpretation there is no obligation on the doctor even to see, let alone examine the body before issuing the certificate. The Broderick report recommended that a doctor should be required to inspect the body of a deceased person before issuing the certificate but this recommendation has never been implemented. Thus, there is no requirement in English law for a general practitioner or any other registered medical practitioner to see or examine the body of a person who is said to be dead.
|
|
|
Sudden or unexpected deaths
These fall into two main categories?. |
Sudden or unexpected deaths
These fall into two main categories: (i) deaths where there is prima facie evidence of violence or other unnatural causes, including deaths in road traffic accidents, falls from high places, suicides and those apparently involving criminal violence; and (ii) sudden or unexpected death where there is no prima facie evidence of violence or unnatural causes. GPs are advised to be cautious in making or attempting to make this distinction unless they are forensically trained and experienced in clinical forensic medicine. It is too easy to wrongly classify a sudden or unexpected death. |
|
|
In practice, the wise practitioner will report a sudden death to ?
|
As a citizen, a doctor has an obligation to inform the police if he/she becomes aware of a serious crime but English law, contrary to popular belief, does NOT, at present, place an obligation upon a doctor to report all sudden deaths to the coroner. In practice, the wise practitioner will report a sudden death to the coroner, normally through the agency of the local police.
The most likely circumstances in which GPs may be requested to attend upon the body of a victim of sudden death are: (i) A call from a relative or a nursing or residential home, about a registered patient who has been found to be dead, unexpectedly, but apparently in circumstances which are not suspicious. The GP, or OOH GP, should respond as quickly as the urgent needs of their living patients permit. On arrival the doctor should carry out an adequate examination to confirm death and then consider whether the coroner should be informed. In all but very exceptional circumstances, even where there appear to be no suspicious circumstances, the doctor would be wise to notify the coroner. When an OOH doctor attends, they OOH organisation have a duty to inform the practice at which the deceased is registered. The GP should be mindful of the considerable distress this may cause to relatives and friends and explain why the police will attend and the likely course of events subsequent to the attendance of the police. (ii) A request from the police, or ambulance service that the GP attend upon a body found in a public place, a deserted building or as the result of a road or other form of accident or other situation. In these circumstances there is no obligation upon the GP to attend. Under the Regulations and Directions underpinning the various contractual arrangements for primary medical services an NHS GP is required to provide treatment to persons not registered but requiring immediate treatment due to an accident or other emergency only if “he is available to provide such treatment”. If the request is to attend upon a dead person or persons there is no question of a GP being requested to provide treatment, therefore there is no obligation to attend. If the request is to attend to treat a person as a result of an accident it may be that the GP, whether the call is in working hours or out of working hours, is available and considers it would be appropriate to attend and not endanger the other patients for whom he/she is responsible to attend the emergency. It would then be right and reasonable for the doctor to attend. However, if the doctor is on call and dealing with numerous calls as when on duty for a co-operative or dealing with patients attending a surgery session, then it is reasonable to give a reply which indicates that the doctor is not available to provide such treatment. If the police request a GP to attend a sudden death, unless that doctor is trained and experienced in clinical forensic medicine and the police offer the appropriate fee for the service, then the GP would be well advised to refuse to attend and advise the police to obtain the services of a retained police surgeon. If the request comes from the ambulance service then the response should be to advise the ambulance service that a doctor is not available and suggest that they ask the police to enlist the services of a retained police surgeon. 3 |
|
|
Cremation Form 4 (formerly cremation form B)
Cremation Form 4 is usually completed by ? |
Cremation Form 4 (formerly cremation form B)
Cremation Form 4 is usually completed by the ordinary medical attendant in charge of the deceased at the time of death. This is often the GP, or the doctor in charge of care during a hospital stay of 24 hours or more. It is important that all parts of form 4 are completed accurately to ensure that the body is released in a timely fashion and that there are no queries about the death following the cremation. |
|
|
When is Form 5 required (formerly cremation form C)?
|
When is Form 5 required (formerly cremation form C)?
A Form 5 is required to corroborate the medical circumstances of a death as stated by a medical practitioner in Form 4. |
|
|
Eligibility to Sign Cremation Form C
Regulation 9 of the Cremation Regulations states that in order to be eligible to complete cremation Form 5, you must be ? |
Eligibility to Sign Cremation Form C
Regulation 9 of the Cremation Regulations states that in order to be eligible to complete cremation Form 5, you must be a “registered medical practitioner of not less than five years' standing.”2 The Department for Constitutional Affairs guidance on this subject goes on to state that “This requires a continuous period of registration at the relevant time. As far as limited registration is concerned, periods of temporary or provisional registration would not seem to disqualify a registered doctor from completing a confirmatory certificate, but it will be a matter for the medical referee to decide whether an inadequate length of full registration may be a factor to be taken into account in any particular case.”3 The medical practitioner who completes the confirmatory medical certificate should not be a relative of the deceased, or a partner of the doctor who has given the cremation certificate in Form 4. Locums and former partners are permitted to complete cremation Form 5, however, we would advise these doctors not to complete cremation Form 5 for practices where they regularly or have recently worked. |
|
|
A confirmatory medical certificate is not required for cremation where ? (2)
|
A confirmatory medical certificate is not required where—
(a) the death of the deceased person occurred in a hospital in which the deceased person was an in-patient; and (b) a medical practitioner mentioned in paragraph (2) has made or supervised a post-mortem examination of the body of the deceased person and the medical practitioner giving the medical certificate (in accordance with paragraph (1)) knows the result of that examination before giving that certificate. |
|
|
Deaths due to acute or chronic poisoning, by any substance, and deaths involving drug dependence or misuse of substances other than what must be referred.
|
Deaths due to acute or chronic poisoning, by any substance, and deaths involving drug dependence or misuse of substances other than alcohol and tobacco must be referred.
|
|
|
Risk factors for meningitis?
|
Risk factors for meningitis include the following:
• Age of 60 years or greater • Age of 5 years or less • Diabetes mellitus, renal or adrenal insufficiency, hypoparathyroidism, or cystic fibrosis • Immunosuppression, which increases the risk of opportunistic infections and acute bacterial meningitis • Human immunodeficiency virus (HIV) infection, which predisposes to bacterial meningitis caused by encapsulated organisms, primarily S pneumoniae, and opportunistic pathogens • Crowding (eg, military recruits and college dorm residents), which increases the risk of outbreaks of meningococcal meningitis • Splenectomy and sickle cell disease, which increase the risk of meningitis secondary to encapsulated organisms • Alcoholism and cirrhosis • Recent exposure to others with meningitis, with or without prophylaxis • Contiguous infection (eg, sinusitis) • Dural defect (eg, traumatic, surgical, congenital) • Thalassemia major • Intravenous (IV) drug abuse • Bacterial endocarditis • Ventriculoperitoneal shunt • Malignancy (increased risk of Listeria species infection) • Some cranial congenital deformities |
|
|
Acute bacterial meningitis - common causative organisms?
|
Acute bacterial meningitis
Bacterial meningitis - An acute and dangerous form of the disease associated with classical symptoms. Common bacteria that cause meningitis depends on the age of the patient. Infants are commonly affected by Streptococcus pneumonia, Listeria, E. coli and Hemophilus influenzae. Meningococcus (Neisseria meningitidis) is the commonest causative in adolescents and middle aged individuals, while Streptococcus pneumonia is again the most common causative bacterial organism causing meningitis in the elderly. Mycobacterium are also a causative of meningitis. Acute bacterial meningitis denotes a bacterial cause of this syndrome. This is usually characterized by an acute onset of meningeal symptoms and neutrophilic pleocytosis. Depending on the specific bacterial cause, the syndrome may be called, for example, any of the following: • Pneumococcal meningitis • Haemophilus influenzae meningitis • Staphylococcal meningitis • Meningococcal meningitis • Tuberculous meningitis Unlike subacute (1-7 d) or chronic (>7 d) meningitis, which have myriad infectious and noninfectious etiologies, acute meningitis (< 1 d) is almost always a bacterial infection caused by 1 of several organisms. Depending on age and general condition, these gravely ill patients present acutely with signs and symptoms of meningeal inflammation and systemic infection of less than 24 hours' duration (and usually >12 hours’ duration). Patients with acute bacterial meningitis may decompensate very quickly, and so they require emergency care, including antimicrobial therapy, ideally within 30 minutes of emergency department (ED) presentation. |
|
|
Fungal meningitis - common causative organisms?
|
Fungal - Cryptococcal meningitis is a serious and fatal form of the disease in patients with HIV/AIDS and a CD count of <200. Candida and aspergillus are other examples of fungi associated with meningitis.
|
|
|
Viral meningitis cammon causes?
|
Viral meningitis - The most common but less serious form of meningitis. Enteroviruses are the most common viral cause of meningitis in the US. Coxsackie, herpes virus, arbovirus, measles and varicella are other common meningitis causing viruses.
|
|
|
Parasitic meningitis cammon causes?
|
Parasites such as Nigleria fowleri and Acanthamoeba species are also etiologic agents of meningitis.
|
|
|
Viral meningitis cammon causes?
|
Viral meningitis - The most common but less serious form of meningitis. Enteroviruses are the most common viral cause of meningitis in the US. Coxsackie, herpes virus, arbovirus, measles and varicella are other common meningitis causing viruses.
|
|
|
Parasitic meningitis cammon causes?
|
Parasites such as Nigleria fowleri and Acanthamoeba species are also etiologic agents of meningitis.
|
|
|
■ CSF opening pressure ranges from?
|
■CSF pressure ranges from 8-10 cm water
|
|
|
normal CSF findings?
■ CSF pressure ? ■ RBC's ? ■ WBC's ? ■ protein ? ■ glucose CSF:blood ratio? ■ glucose ? |
normal findings
■ CSF pressure ranges from 8-10 cm water ■ RBC's = 0 ■ WBC's =< 5 cells/microL (< 20 lymphocytes/microL in neonates) ■ The median CSF WBC count was significantly higher in infants who were aged ≤28 days (3/μL, 95th percentile: 19/μL) than in infants who were aged 29 to 56 days (2/μL, 95th percentile: 9/μL; P < .001)1) ■ protein: < 0.4g/L (< 1g/L in neonates) ■ glucose CSF:blood ratio >= 0.6 ■ glucose >= 2.5mM |
|
|
Typical CSF findings in acute bacterial meningitis?
|
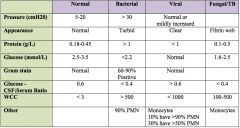
|
|
|
Typical CSF findings in acute fungal meningitis?
|
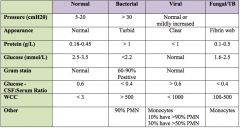
|
|
|
Typical CSF findings in TB meningitis?
|
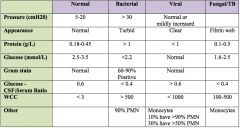
|
|
|
Abusive head trauma (AHT) presents with ?
|
Abusive head trauma (AHT) is the leading cause of death from traumatic brain
injury in under 2 year olds. AHT presents with acute encephalopathy, subdural hemorrhages and retinal hemorrhages occurring in the context of an inappropriate or inconsistent history. |
|
|
Children with suspected abusive head trauma should undergo appropriate investigations which should
include ? |
Children with suspected AHT should undergo appropriate investigations which should
include brain imaging, ophthalmic examination, skeletal survey and blood investigations. Early social work assessment is a priority as part of the multidisciplinary approach. |
|
|
Cellulitis - The vast majority of cases are caused by? (2)
|
Cellulitis usually follows a break in the skin, such as a fissure, cut, laceration, insect bite, or puncture wound. Organisms on the skin and its appendages gain entrance to the dermis and multiply to cause cellulitis. Facial cellulitis of odontogenic origin may also occur. However, cellulitis frequently occurs in areas where no apparent injury exists. This is common in dry and irritated skin where microscopic breaks allow penetration of bacteria. Patients with toe web intertrigo and/or tinea pedis and those with lymphatic obstruction, venous insufficiency, pressure ulcers, and obesity are particularly vulnerable to recurrent episodes of cellulitis.[1, 2, 3, 4]
The vast majority of cases are caused by Streptococcus pyogenes or Staphylococcus aureus. Occasionally, cellulitis may be caused by the emergence of subjacent osteomyelitis. Cellulitis may rarely result from the metastatic seeding of an organism from a distant focus of infection, especially in immunocompromised individuals. This is particularly common in cellulitis due to S pneumoniae (pneumococcus) and marine vibrios. Neisseria meningitidis, Pseudomonas aeruginosa, Brucella species, and Legionella species have also been reported as rare causes of cellulitis resulting from hematogenous spread.[ |
|
|
Certain host factors predispose to severe infection ?
|
Certain host factors predispose to severe infection. The elderly and individuals with diabetes mellitus or hypertension are at risk for more severe disease.[6] Patients with diabetes, immunodeficiency, cancer, venous stasis, chronic liver disease, peripheral arterial disease, and chronic kidney disease appear to be at a higher risk for recurrent infection, owing to an altered host immune response. Other factors that affect host immunity and predispose to cellulitis include concurrent intravenous (IV) or subcutaneous (SC) “skin popping” drug use; infections in this setting may be polymicrobial, but methicillin-resistant S aureus (CA-MRSA) is the most common pathogen in these patients.
In individuals with normal host defenses, the most common causative organisms are group A streptococci (GAS) and S aureus. Group B Streptococcus cellulitis occurs in infants younger than 6 months, as their immune responses are not fully developed. The cellulitis may present as sepsis[7] ; underlying osteomyelitis or septic arthritis must be excluded in these infants. (See the image below.) A case of cellulitis without associated purulence in an infant. Note the presence of lymphedema, a risk factor for cellulitis (Photo courtesy of Amy Williams). In children, facial cellulitis is frequently associated with H influenzae type B and S pneumoniae; prophylactic pneumococcal vaccine may be effective in the latter cases. A study of one-half million pediatric hospitalizations demonstrated that, although bacterial meningitis and epiglottitis diminished as a result of immunization for H influenzae type B and S pneumoniae, the incidence of facial cellulitis was unaffected.[8] However, another study noted that 96% of the serotypes that cause facial cellulitis are included in the heptavalent-conjugated pneumococcal vaccine that is licensed in the United States. Immunocompromised hosts typically become infected from opportunistic organisms, including gram-negative rods (eg, Pseudomonas, Proteus, Serratia, Enterobacter, Citrobacter), anaerobes, and others (eg, Helicobacter cinaedi, Fusarium species). Although fungi (eg, Cryptococcus) may also cause cellulitis, this is rare. Pneumococci may cause a particularly malignant form of cellulitis that is frequently associated with tissue necrosis, suppuration, and bloodstream invasion. Two distinct syndromes are recognized: the first is marked by involvement of the extremities in patients with diabetes or substance abuse, and the second is marked by involvement of the head, neck, and upper torso in patients with systemic lupus erythematosus, nephrotic syndrome, or hematologic disorders.[9] Mycobacterial infections may present as cellulitis. Typically, the lack of response to antibiotics prompts further investigation. The diagnosis is made based on the presence of granulomas, multinucleated giant cells, and acid-fast bacilli (AFB) from biopsy specimens.[10, 11, 12] S aureus is the leading cause of soft-tissue infections in injection drug users,[13] followed by Streptococcus species.[14] Gram-negative bacteria may cause bullous cellulitis in patients with cirrhosis.[15] Early recognition is vital, as the course of the disease is rapid, typically progressing to septic shock and death. Gram stain and culture of fluid aspirated from the bullae may aid in management. Recurrent staphylococcal cellulitis may occur in patients with nasal carriage of staphylococci and those with Job syndrome. |
|
|
Cellulitis: 4 cardinal signs of infection?
|
4 cardinal signs of infection: erythema, pain, swelling, and warmth. Several physical examination findings may help the clinician to identify the most likely pathogen and to assess the severity of the infection, facilitating appropriate treatment, including the following:
• The involved sites are red, hot, swollen, and tender. • Unlike erysipelas, the borders are not elevated or sharply demarcated. • The most commonly involved site is the leg.[31, 46] • Regional lymphadenopathy may be present. • Malaise, chills, fever, and toxicity may occur. • Skin infection without underlying drainage, penetrating trauma, eschar, or abscess is most likely caused by streptococci; on the other hand, S aureus, often community-acquired MRSA (CA-MRSA), is the most likely pathogen when these factors are present.[47] • Perianal cellulitis is usually observed among children with perianal fissures; it is characterized by perianal erythema and pruritus, purulent secretions, painful defecation, and bleeding in the stools.[48] • Cellulitis characterized by violaceous color and bullae suggests systemic infection with organisms such as V vulnificus or S pneumoniae. • Lymphangitic spread (red lines streaking away from the area of infection), crepitus, and hemodynamic instability are indications of severe infection, requiring more aggressive treatment. • Circumferential cellulitis or pain that is disproportional to examination findings should prompt consideration of more severe soft-tissue infection. |
|
|
Finally, consider an alternative diagnosis that might be commonly confused with cellulitis. Differentials ?
|
Diagnostic studies are generally unnecessary in uncomplicated cellulitis, and most cases respond well to standard antibiotic regimens. If there is no response to the initial choice of antibiotic, the organism may be resistant to the drug. Also, consider unusual organisms that may require combinations of antibiotics. Finally, consider an alternative diagnosis that might be commonly confused with cellulitis. See the Differentials section for a complete list of articles on other conditions to consider.
Other conditions that should be considered include the following: • Necrotizing fasciitis • Anaerobic myonecrosis • Calciphylaxis • Cutaneous anthrax • Cutaneous metastasis from neoplasms (especially adenocarcinoma) • Envenomation by puncture with spines of stonefish (in the South Pacific) • Familial Mediterranean fever • Graft versus host disease • Hyperimmunoglobulin D syndrome • Inflammatory carcinoma of the breast • Neutrophilic eccrine hidradenitis • “Seal finger” secondary to seal bites (in aquarium workers and veterinarians)[49] • Sweet syndrome[50] • Tumor necrosis factor receptor-associated syndrome Differentials •Burn Wound Infections •Erysipelas •Erysipeloid •Erythema Multiforme •Gas Gangrene •Insect Bites •Leukemia Cutis •Lymphoma, Cutaneous T-Cell •Mycosis Fungoides •Myiasis •Necrotizing Fasciitis •Nocardiosis •Pyoderma Gangrenosum •Stevens-Johnson Syndrome •Wells Syndrome (Eosinophilic Cellulitis) |
|
|
Differentials for orbital cellulitis?
|
Differentials
•Exophthalmos •Mucormycosis •Retinoblastoma •Sarcoidosis •Spider Bites •Thyroid Ophthalmopathy |
|
|
Risk factors for suicide?
|
Risk factors for suicide
•Male gender (3 times more likely than women) •Advancing age •Unemployed •Concurrent mental disorders •Previous suicide attempt •Alcohol and drug abuse •Low socio-economic status •Previous psychiatric treatment •Certain professions - doctors, students •Low social support / living alone •Significant life events •Institutionalised e.g. prisons, army |
|
|
PATHOS - Self-harm assessment?
|
PATHOS - Self-harm assessment
'Have you had Problems for longer than 1 month?' 'Were you Alone in the house when you overdosed?' 'Did you plan the overdose for more than Three hours?' Are you feeling HOpeless about the future - that things will not get much better?' 'Were you feeling Sad for most of the time before the overdose?' The more features present - the greater the likelihood of significant suicidal intent and depression |
|
|
Management after initial assessment for suicide risk?
|
Management after initial assessment
•If the patient is at low risk then they should be offered regular contact (could be by telephone if possible) and counselling. You may need to consider referral to local mental health services for further follow-up. •If there are concerns about patient safety or the patient scores highly on the suicide risk score the patient should be referred for urgent mental health assessment. If you are unsure then seek advice from mental health specialists.2,8 •Usually patients are sent to a designated assessment area and the on-call psychiatrist can direct you as required. One needs to be wary of sending patients to A&E, although most A & E departments have psychiatric liaison staff available on site allowing the patient to be assessed and admitted if necessary. •If a patient refuses help then a decision regarding their capacity may need to be made with psychiatric evaluation and detention under the Mental Capacity Act considered.8 •It is important to remember that scales of risk, although helpful, have a poor predictive value. Therefore, if you have a patient who you are worried about but they score low, then still consider urgent referral for them. |
|
|
From which month the fetus also begins to drink amniotic fluid ? How much?
|
Physiology of the amniotic fluid
Amniotic fluid is to be found in the amniotic cavity. It completely surrounds the embryo after the 4th week of pregnancy. In this way it insures freedom of movement for the embryo, space for development, absorbs blows, and keeps the embryo from sticking to the placenta. Towards the outside, the amniotic cavity is delimited by the amniotic epithelium, the chorion laeve and the decidua capsularis. This is the interface to the maternal compartment. The amniotic fluid is a clear, watery fluid that is filtered out of the maternal blood via the amniotic epithelium into the amniotic cavity. A large portion stems also from the fetus itself (from the skin, the umbilical cord, the lungs and the kidneys). The makeup of the amniotic fluid is thus quite complex, with many maternal and fetal constituents. The main constituents are water and electrolytes (99%) together with glucose, lipids from the fetal lungs, proteins with bactericide properties and flaked-off fetal epithelium cells (they make a prenatal diagnosis of the infantile karyotype possible). Its quantity changes over the course of the pregnancy (20 ml in the 7th week, 600 ml in the 25th week, 1000 ml in the 30th to 34th week and 800 ml at birth). From the 5th month the fetus also begins to drink amniotic fluid (400 ml/day). Close to the end of the pregnancy the amniotic fluid is replaced all 3 hours, stressing the importance of this exchange between the amniotic fluid and the maternal compartment. |
|
|
Questions for Evaluating Hearing Loss?
|
Questions for Evaluating Hearing Loss
-------------------------------------------------------------------------------- When did your hearing loss begin? Was your hearing loss sudden, or has your hearing slowly been getting worse? Does your hearing loss involve one or both ears? Have you been having ringing in your ear, fullness in your ear, dizziness, ear drainage, or ear pain? Is there a history of hearing loss in your family? What is your job? What is the noise level in your workplace? Do you have a history of ear infections, ear injury, or straining to hear? Do you have a history of stroke, diabetes, or heart disease? What medicines are you currently taking? Have you received any intravenous antibiotics, diuretics, salicylates, or chemotherapy? |
|
|
Otitis externa is ?
|
Otitis externa is an infection of the skin of the external auditory canal. Patients with otitis externa experience pain on manipulation of the pinna or tragus, and their ear canal is edematous and filled with infectious debris. Conductive hearing loss may occur if swelling and debris occlude the canal. The most common pathogens in otitis externa are Pseudomonas aeruginosa and Staphylococcus aureus.4 Treatment involves debridement of the canal, followed by the application of ototopical drops. In patients with severe otitis externa, a wick is placed in the ear for two to three days to ensure delivery of the medication. Oral antibiotics that are effective against P. aeruginosa and S. aureus are helpful in patients with severe infection. The conductive hearing loss resolves after the inflammation subsides.
|
|
|
Exostoses and osteomas are ?
|
Exostoses and osteomas are benign bony growths of the external auditory canal that interfere with normal cerumen migration, leading to occlusion and conductive hearing loss. Exostoses are multiple and bilateral, and are found adjacent to the tympanic membrane. Patients with exostoses often report a history of cold-water swimming. Osteomas are single and unilateral, and are found at the bony-cartilaginous junction (Figure 2). If symptomatic, exostoses and osteomas are removed surgically, but this is rarely necessary.
|
|
|
Uncommon causes of external auditory canal obstruction include cysts and tumors. Sebaceous cysts, fibromas, papillomas, adenomas, sarcomas, carcinomas, and melanomas also have been reported. If a malignancy is suspected, what is indicated?
|
Uncommon causes of external auditory canal obstruction include cysts and tumors. Sebaceous cysts, fibromas, papillomas, adenomas, sarcomas, carcinomas, and melanomas also have been reported. If a malignancy is suspected, prompt biopsy is indicated.
|
|
|
Middle ear pathology may lead to what type of hearing loss?
|
Middle ear pathology may lead to conductive hearing loss. Perforations of the tympanic membrane cause hearing loss by reducing the surface area available for sound transmission to the ossicular chain
|
|
|
The main causes of tympanic membrane perforations are ?
|
The main causes of tympanic membrane perforations are chronic otitis media and trauma. In patients who have had chronic otitis media with tympanic membrane perforation, otoscopic examination and debridement are essential. Ototopical antibiotics (ofloxacin [Floxin]) are necessary, and oral antibiotics may be helpful. An accurate assessment of the patient's tympanic membrane and hearing can be made only when the ear is dry.
|
|
|
Traumatic perforations of the tympanic membrane can occur because of ?
|
Traumatic perforations of the tympanic membrane can occur because of water accidents, barotrauma, explosions, penetrating injury, or temporal bone fractures. Small perforations (less than 2 mm) often heal spontaneously.5 In the acute setting, blood may obstruct the ear canal and prevent visualization of the membrane. Ototopical antibiotics and precautions to keep the ear dry are recommended. If the perforation or hearing loss persists beyond two months, the patient should be referred for consideration of surgical correction. Trauma also can cause ossicular injury or hemotympanum presenting as hearing loss.
|
|
|
Most common cause of conductive hearing loss in children?
|
Otitis media is the most common cause of conductive hearing loss in children.6 Middle ear effusions decrease the mobility of the tympanic membrane and the ossicular chain. This loss of mobility results in an average hearing loss of 20 to 30 dB. The diagnosis of otitis media can be confirmed by tympanometry and audiometry, and resolution of the effusion restores hearing. Myringotomy tubes are recommended for use in children with recurrent acute otitis media (more than three episodes in six months or four episodes in one year), chronic middle ear effusions (more than three months in duration), or significant hearing impairment (greater than 30 dB along with an effusion).7 [Evidence level C, consensus opinion]
|
|
|
Cholesteatoma is ?
|
Cholesteatoma is an accumulation of squamous epithelium within the middle ear. This mass may be seen in patients with otitis media. Cholesteatomas are divided into two types: congenital and acquired. Congenital cholesteatoma presents as a pearly white mass located behind an intact tympanic membrane in a patient with unilateral conductive hearing loss. Acquired cholesteatoma results from a retracted or perforated tympanic membrane with an ingrowth of epithelium. Cholesteatomas are locally destructive and characterized by chronic drainage. Conductive hearing loss caused by ossicular erosion is present in 90 percent of patients with cholesteatomas.8 Longstanding cholesteatomas expand to involve the mastoid, inner ear, and facial nerve. Suspicion of cholesteatoma warrants surgical consultation.
|
|
|
Myringosclerosis of the tympanic membrane develops in response to ?
|
Myringosclerosis of the tympanic membrane develops in response to infection or inflammation (Figure 3). Irregular white patches consisting of calcium are visible on the membrane.9 Isolated myringosclerosis of the tympanic membrane rarely causes significant conductive hearing loss. However, extensive myringosclerosis, referred to as tympanosclerosis, involves the tympanic membrane, ossicular chain, and middle ear mucosa, and causes significant conductive hearing loss by stiffening the entire system.
|
|
|
Otosclerosis is characterized by ?
|
Otosclerosis is characterized by abnormal bone deposition at the footplate (base of stapes). This bone deposition leads to fixation of the stapes at the oval window, preventing normal vibration. Otosclerosis typically presents as progressive bilateral conductive hearing loss in middle-aged white women. It is the leading cause of conductive hearing loss in adults who do not have a middle ear effusion or a history of otitis media.10 There is usually a positive family history. Treatment consists of amplification with hearing aids or surgical repair by stapedectomy
|
|
|
Glomus tumors are a rare cause of what type of hearing loss?
|
Glomus tumors are a rare cause of conductive hearing loss (Figure 5). These neuroendocrine tumors arise from the adventitia of the jugular bulb or the neural plexus within the middle ear space. Characteristically, patients presenting with glomus tumors are women 40 to 50 years of age who report pulsatile tinnitus and hearing loss. On examination, a pulsating reddish-blue mass may be seen behind an intact tympanic membrane. However, diagnosis of these tumors is difficult, and computed tomography of the temporal bones is required. An anomalous carotid artery or jugular bulb may present in a similar fashion.
|
|
|
High-Risk Indicators* of Hearing Loss in Infants and Young Children?
|
High-Risk Indicators* of Hearing Loss in Infants and Young Children
-------------------------------------------------------------------------------- Birth to 28 days Family history of permanent sensorineural hearing loss during childhood In utero infection (e.g., toxoplasmosis, rubella, cytomegalovirus infection, herpes) Ear or other craniofacial abnormalities Illness or condition requiring admission to neonatal intensive care unit for at least 48 hours Physical features or other stigmata associated with a syndrome known to include sensorineural or conductive hearing loss |
|
|
High-Risk Indicators* of Hearing Loss in Infants and Young Children
29 days to 24 months? |
High-Risk Indicators* of Hearing Loss in Infants and Young Children
-------------------------------------------------------------------------------- Birth to 28 days Family history of permanent sensorineural hearing loss during childhood In utero infection (e.g., toxoplasmosis, rubella, cytomegalovirus infection, herpes) Ear or other craniofacial abnormalities Illness or condition requiring admission to neonatal intensive care unit for at least 48 hours Physical features or other stigmata associated with a syndrome known to include sensorineural or conductive hearing loss 29 days to 24 months Parental or caregiver concern about hearing, speech, language, or developmental delay Family history of permanent hearing loss during childhood Physical features or other stigmata associated with a syndrome known to include sensorineural or conductive hearing loss or eustachian tube dysfunction Head trauma Postnatal infection associated with sensorineural hearing loss (e.g., meningitis) In utero infection (e.g., toxoplasmosis, rubella, cytomegalovirus infection, herpes, syphilis) Neonatal indicators: hyperbilirubinemia requiring exchange transfusion, persistent pulmonary hypertension associated with mechanical ventilation, conditions requiring extracorporeal membrane oxygenation Syndromes associated with progressive hearing loss (e.g., neurofibromatosis, osteopetrosis, Usher's syndrome) Neurodegenerative disorders (e.g., Hunter's syndrome) or sensory motor neuropathies (e.g., Friedreich's ataxia, Charcot-Marie-Tooth disease) Head trauma Recurrent or persistent otitis media with effusion for at least three months |
|
|
What is the most common preventable cause of sensorineural hearing loss?
|
Noise trauma is the most common preventable cause of sensorineural hearing loss. The noise source may be occupational, recreational, or accidental. Gunfire, explosions, and loud music can cause irreversible hearing impairment. High frequencies are affected first, typically at 4,000 Hz, followed by middle and lower frequencies. The hearing loss is accompanied by high-pitched tinnitus. Aggressive use of noise protection is recommended to prevent this form of hearing loss. The use of foam-insert earplugs decreases noise exposure by 30 dB.
|
|
|
Autoimmune hearing loss has been diagnosed with increasing frequency since the 1980s. Patients present with ?
|
Autoimmune hearing loss has been diagnosed with increasing frequency since the 1980s. Patients present with rapidly progressive bilateral sensorineural hearing loss and poor speech discrimination scores, and they also may have vertigo or disequilibrium. Hearing loss progresses over three to four months, and an associated autoimmune disorder may be present. Symptoms usually improve with the administration of oral prednisone, and response to this steroid is currently the best way to make the diagnosis. Low-dose methotrexate therapy is becoming an accepted alternative to long-term prednisone therapy
|
|
|
Temporal bone fractures can cause what type of hearing loss?
|
Temporal bone fractures can cause unilateral sensorineural and conductive hearing loss. When the fracture line involves the bony labyrinth (cochlea or vestibule), sensorineural hearing loss occurs. Temporal bone injuries are associated with facial nerve paralysis, cerebrospinal fluid leakage, and other intracranial injuries. Early consultation is essential, and prompt surgical intervention may be required.
|
|
|
Trauma may cause rupture of the round or oval window membranes, with perilymph leaking into the middle ear (fistula). Patients experience ?
|
Trauma may cause rupture of the round or oval window membranes, with perilymph leaking into the middle ear (fistula). Patients experience abrupt loss of hearing, along with vertigo and tinnitus. Perilymph fistulas also may occur after straining, lifting, coughing, or sneezing, and are managed with three to six weeks of bed rest, followed by surgical repair if symptoms do not improve
|
|
|
Meniere's disease is a cause of what kind of hearing loss?
|
Meniere's disease is a cause of sensorineural hearing loss. Patients report unilateral fluctuating hearing loss with aural fullness, tinnitus, and episodic vertigo. Initially, the hearing loss is in the low frequencies, but higher frequencies are affected as the disease progresses. The etiology of Meniere's disease remains unclear, but endolymphatic hydrops (increased fluid pressure within the inner ear) has been identified. The work-up consists of serial audiometry to document a fluctuating loss, vestibular testing to verify the diseased ear, and radiographic imaging to rule out an acoustic tumor. Treatment includes a low-salt diet, diuretics, and vestibular suppressants. Hearing aids are often ineffective because patients suffer from poor speech discrimination, as well as diminished tolerance to amplified sound. Chemical labyrinthectomy with gentamicin is now a common nonsurgical option for control of vertigo if medical management fails
|
|
|
Patients with acoustic neuromas present with ?
|
Patients with acoustic neuromas present with unilateral sensorineural hearing loss approximately 10 to 22 percent of the time19 (Figure 6). Patients with asymmetric sensorineural hearing loss require evaluation for a retrocochlear tumor. Acoustic neuromas and other cerebellopontine-angle tumors need to be ruled out. Magnetic resonance imaging of the brain with gadolinium continues to be the gold standard for diagnosing these masses.
|
|
|
The spinal cord ends at the interspace between ?
|
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Spinal pathology below L1 presents with ?
|
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Aetiology (6)
|
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Clinical presentation
Clinical features depend on extent and rate of development of cord compression Motor symptoms include? |
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Clinical presentation
Clinical features depend on extent and rate of development of cord compression Lumbar spine disease affects which nerve roots? |
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Clinical presentation
Clinical features depend on extent and rate of development of cord compression Sensory symptoms include ? |
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Cauda equina compression due to central disc prolapse produces ?
|
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
Spinal cord compression - Investigation ?
|
Spinal cord compression
The clinical features of a spinal cord lesion depend on its rate of development Trauma produces acute compression with rapidly developing effects Benign neoplasms can cause substantial compression with little neurological deficit Anatomy The spinal cord is shorter than spinal canal The cord ends at the interspace between the L1 and L2 vertebrae Below the termination of the cord the nerve roots form the cauda equina Within cervical spine segmental levels of cord correspond to bony landmarks Below this level there is increasing disparity between levels Spinal pathology below L1 presents with only root signs Aetiology Trauma - vertebral body fracture or facet joint dislocation Neoplasia - benign or malignant Degenerative - prolapsed intervertebral disc, osteophyte formation Vascular - epidural or subdural haematoma Inflammatory - rheumatoid arthritis Infection - tuberculosis or pyogenic infections Clinical presentation Clinical features depend on extent and rate of development of cord compression Motor symptoms include easy fatigue and gait disturbance Cervical spine disease produces quadriplegia Thoracic spine disease produces paraplegia Lumbar spine disease affects L4, L5 and sacral nerve roots Sensory symptoms include sensory loss and paraesthesia Light touch, proprioception and joint position sense are reduced Tendon reflexes are often: Increased below level of compression Absent at level of compression Normal above level of compression Reflex changes may not coincide with sensory level Sphincter disturbances are late features of cervical and thoracic cord compression Cauda equina compression due to central disc prolapse produces: Loss of perianal sensation 'Root pain' in both legs Painless urinary retention Most patients with surgical treatable causes of spinal compression have spinal pain Movement induce pain suggests vertebral fracture or collapse Exquisite tenderness suggests an epidural abscess Low-grade background pain suggests tumour infiltration or osteomyelitis Investigation Plain x-rays may show bone or paravertebral soft tissue disease Features include vertebral collapse, lytic lesions, loss of pedicle Integrity of disc may indicate diagnosis 'Good disc = bad news' often indicates malignancy 'Bad disc = good news' may indicate infection MRI is investigation of choice to define extent of soft tissue disease Bone scan may indicate pattern and extent of bone pathology Management Acute cord compression is a 'surgical' emergency In those with malignant disease radiotherapy may be treatment of choice In general, tumour, infection and disc disease produces anterior compression Surgical decompression should be achieved through an anterior approach Cervical spine can be approached between larynx medially and carotid sheath laterally Thoracic spine can be approached through chest by a posterior thoracotomy or costotransversectomy |
|
|
%BSA of different regions Region Adult (%) ?
|
Relative %BSA of different regions varies between children and adults
Region Adult (%) Child (%) Head 9 19 Body 18 18 Upper limb 9 9 Lower limb 18 13 Perineum 1 1 |
|
|
%BSA of different regions - Child (%)
|
Relative %BSA of different regions varies between children and adults
Region Adult (%) Child (%) Head 9 19 Body 18 18 Upper limb 9 9 Lower limb 18 13 Perineum 1 1 |
|
|
Diagnostic criteria for non-accidental injury? (13)
|
Diagnostic criteria for non-accidental injury
Delay in seeking medical advice Vague or inconsistent account of the accident Discrepancy between the history and degree of injury Abnormal parental behaviour or lack of concern for the child Interaction between child and parents is abnormal Finger tip bruising over upper arm, trunk, face or neck Bizarre injuries - bites, cigarette burns or rope marks Sharply demarked burns in unusual site Perioral injuries - torn frenulum Retinal haemorrhages Ruptured internal organs without a history of major trauma Perianal or genital injury Long bone fractures in children less than 3 years Injuries of differing ages |
|
|
Circulation in children:
Less than one year? pulse ? systolic BP ? |
Circulation in children:
Normal values for pulse and blood pressure vary with age Less than one year, pulse = 120 to 140 and systolic BP is 70-90 mmHg Between 2 and 5 years, pulse is 100-120 and systolic BP is 80-90 mmHg Between 5 and 12 years, pulse is 80-100 and systolic BP is 90-110 mmHg Venous access in a child can be difficult Femoral or external jugular access may be required If percutaneous cannulation fails need to consider Medial cephalic venous cut down Long saphenous venous cut down Intraosseous infusion Initial resuscitation should be with a 20 ml/kg crystalloid bolus |
|
|
Circulation in children:
Between 2 and 5 years ? pulse ? systolic BP ? |
Circulation in children:
Normal values for pulse and blood pressure vary with age Less than one year, pulse = 120 to 140 and systolic BP is 70-90 mmHg Between 2 and 5 years, pulse is 100-120 and systolic BP is 80-90 mmHg Between 5 and 12 years, pulse is 80-100 and systolic BP is 90-110 mmHg Venous access in a child can be difficult Femoral or external jugular access may be required If percutaneous cannulation fails need to consider Medial cephalic venous cut down Long saphenous venous cut down Intraosseous infusion Initial resuscitation should be with a 20 ml/kg crystalloid bolus |
|
|
Circulation in children:
Normal values for pulse and blood pressure vary with age Between 5 and 12 years ? pulse ? systolic BP ? |
Circulation in children:
Normal values for pulse and blood pressure vary with age Less than one year, pulse = 120 to 140 and systolic BP is 70-90 mmHg Between 2 and 5 years, pulse is 100-120 and systolic BP is 80-90 mmHg Between 5 and 12 years, pulse is 80-100 and systolic BP is 90-110 mmHg Venous access in a child can be difficult Femoral or external jugular access may be required If percutaneous cannulation fails need to consider Medial cephalic venous cut down Long saphenous venous cut down Intraosseous infusion Initial resuscitation should be with a 20 ml/kg crystalloid bolus |
|
|
Differentials for Graves' disease?
|
Differentials
•Anxiety Disorders •Hashimoto Thyroiditis •Hyperemesis Gravidarum •Pheochromocytoma •Pituitary Macroadenomas •Pituitary Microadenomas •Struma Ovarii •Subacute Thyroiditis •Thyroid, Papillary Carcinoma •Thyrotropin-producing pituitary adenomas •Toxicity, Cocaine •Wolff-Parkinson-White Syndrome |
|
|
Both men and women can get Graves' disease. But it affects women 10 times more often than men. Graves' disease occurs in people of all ages, but most often starts in the 20s and 30s. People who get Graves' disease often have family members who have thyroid or other autoimmune diseases. People who get Graves' disease sometimes have other autoimmune diseases, such as ?
|
Both men and women can get Graves' disease. But it affects women 10 times more often than men. Graves' disease occurs in people of all ages, but most often starts in the 20s and 30s. People who get Graves' disease often have family members who have thyroid or other autoimmune diseases. People who get Graves' disease sometimes have other autoimmune diseases, such as:
Vitiligo (vit-ihl-EYE-goh) — a disease that destroys the cells that give your skin its color Rheumatoid arthritis — a disease that affects the lining of the joints throughout the body Addison's disease — a disease that affects the adrenal glands, which make hormones that help your body respond to stress and regulate your blood pressure and water and salt balance Type 1 diabetes — a disease that causes blood sugar levels to be too high Pernicious (pur-NISH-uhss) anemia — a disease that keeps your body from absorbing vitamin B12 and making enough healthy red blood cells Lupus — a disease that can damage many parts of the body, such as the joints, skin, blood vessels, and other organs |
|
|
Full antibiotic treatment cures chlamydia STDs and can prevent complications. In woman who have chlamydia PID and remain untreated, government health experts estimate that what percent become infertile, what percent suffer long-term pelvic pain, and what percent eventually have a tubal pregnancy?
|
Full antibiotic treatment cures chlamydia STDs and can prevent complications. In woman who have chlamydia PID and remain untreated, government health experts estimate that 20 percent become infertile, 18 percent suffer long-term pelvic pain, and 9 percent eventually have a tubal pregnancy. Untreated chlamydia infections in men can cause swollen and tender testicles.
|
|
|
Untreated chlamydia infections in men can cause ?
|
Full antibiotic treatment cures chlamydia STDs and can prevent complications. In woman who have chlamydia PID and remain untreated, government health experts estimate that 20 percent become infertile, 18 percent suffer long-term pelvic pain, and 9 percent eventually have a tubal pregnancy. Untreated chlamydia infections in men can cause swollen and tender testicles.
|
|
|
Overall, the changes in cardiac function associated with heart failure result in a decrease in cardiac output. This results from a decline in what ?
|
Overall, the changes in cardiac function associated with heart failure result in a decrease in cardiac output. This results from a decline in stroke volume that is due to systolic dysfunction, diastolic dysfunction, or a combination of the two.
|
|
|
Cardiac and Vascular Changes
Accompanying Heart Failure Cardiac ? (5) |
Cardiac and Vascular Changes
Accompanying Heart Failure Cardiac •Decreased stroke volume & cardiac output •Increased end-diastolic pressure •Ventricular dilation or hypertrophy •Impaired filling (diastolic dysfunction) •Reduced ejection fraction (systolic dysfunction) Vascular •Increased systemic vascular resistance •Decresed aterial pressure •Impaired arterial pressure •Impaired organ perfusion •Decreased venous compliance •Increased venous pressure •Increased blood volume |
|
|
Cardiac and Vascular Changes
Accompanying Heart Failure - Vascular ? (7) |
Cardiac and Vascular Changes
Accompanying Heart Failure Cardiac •Decreased stroke volume & cardiac output •Increased end-diastolic pressure •Ventricular dilation or hypertrophy •Impaired filling (diastolic dysfunction) •Reduced ejection fraction (systolic dysfunction) Vascular •Increased systemic vascular resistance •Decresed aterial pressure •Impaired arterial pressure •Impaired organ perfusion •Decreased venous compliance •Increased venous pressure •Increased blood volume |
|
|
Diastolic dysfunction refers to ?
|
Diastolic dysfunction refers to the diastolic properties of the ventricle and occurs when the ventricle becomes less compliant (i.e., "stiffer"), which impairs ventricular filling.
|
|
|
Systolic cardiac dysfunction results from ? (2)
|
systolic dysfunction results from a loss of intrinsic inotropy (contractility), most likely due to alterations in signal transduction mechanisms responsible for regulating inotropy. Systolic dysfunction can also result from the loss of viable, contracting muscle as occurs following acute myocardial infarction.
|
|
|
Both systolic and diastolic dysfunction result in a higher what ?
|
Both systolic and diastolic dysfunction result in a higher ventricular end-diastolic pressure, which serves as a compensatory mechanism by utilizing the Frank-Starling mechanism to augment stroke volume.
|
|
|
Neurohumoral responses to cardiac failure include ?
|
Neurohumoral responses to cardiac failure include activation of sympathetic nerves and the renin-angiotensin system, and increased release of antidiuretic hormone (vasopressin) and atrial natriuretic peptide. The net effect of these neurohumoral responses is to produce arterial vasoconstriction (to help maintain arterial pressure), venous constriction (to increase venous pressure), and increased blood volume. In general, these neurohumoral responses can be viewed as compensatory mechanisms, but they can also aggravate heart failure by increasing ventricular afterload (which depresses stroke volume) and increasing preload to the point where pulmonary or systemic congestion and edema occur. Therefore, it is important to understand the pathophysiology of heart failure because it serves as the rationale for drug therapy.
There is also evidence that other factors such as nitric oxide and endothelin (both of which are increased in heart failure) may play a role in the pathogenesis of heart failure. Some drug treatments for heart failure involve attenuating the neurohumoral changes. For example, certain beta-blockers have been shown to provide significant long-term benefit, quite likely because they block the effects of excessive sympathetic activation on the heart. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists are commonly used to treat heart failure by inhibiting the actions of the renin-angiotensin-aldosterone system. |
|
|
Neurohumoral responses to cardiac failure include activation of sympathetic nerves and the renin-angiotensin system, and increased release of antidiuretic hormone (vasopressin) and atrial natriuretic peptide. The net effect of these neurohumoral responses is to produce what ?
|
Neurohumoral responses to cardiac failure include activation of sympathetic nerves and the renin-angiotensin system, and increased release of antidiuretic hormone (vasopressin) and atrial natriuretic peptide. The net effect of these neurohumoral responses is to produce arterial vasoconstriction (to help maintain arterial pressure), venous constriction (to increase venous pressure), and increased blood volume. In general, these neurohumoral responses can be viewed as compensatory mechanisms, but they can also aggravate heart failure by increasing ventricular afterload (which depresses stroke volume) and increasing preload to the point where pulmonary or systemic congestion and edema occur. Therefore, it is important to understand the pathophysiology of heart failure because it serves as the rationale for drug therapy.
There is also evidence that other factors such as nitric oxide and endothelin (both of which are increased in heart failure) may play a role in the pathogenesis of heart failure. Some drug treatments for heart failure involve attenuating the neurohumoral changes. For example, certain beta-blockers have been shown to provide significant long-term benefit, quite likely because they block the effects of excessive sympathetic activation on the heart. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists are commonly used to treat heart failure by inhibiting the actions of the renin-angiotensin-aldosterone system. |
|
|
Neurohumoral responses to cardiac failure - can aggravate heart failure by ?
|
Neurohumoral responses to cardiac failure include activation of sympathetic nerves and the renin-angiotensin system, and increased release of antidiuretic hormone (vasopressin) and atrial natriuretic peptide. The net effect of these neurohumoral responses is to produce arterial vasoconstriction (to help maintain arterial pressure), venous constriction (to increase venous pressure), and increased blood volume. In general, these neurohumoral responses can be viewed as compensatory mechanisms, but they can also aggravate heart failure by increasing ventricular afterload (which depresses stroke volume) and increasing preload to the point where pulmonary or systemic congestion and edema occur. Therefore, it is important to understand the pathophysiology of heart failure because it serves as the rationale for drug therapy.
There is also evidence that other factors such as nitric oxide and endothelin (both of which are increased in heart failure) may play a role in the pathogenesis of heart failure. Some drug treatments for heart failure involve attenuating the neurohumoral changes. For example, certain beta-blockers have been shown to provide significant long-term benefit, quite likely because they block the effects of excessive sympathetic activation on the heart. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and aldosterone receptor antagonists are commonly used to treat heart failure by inhibiting the actions of the renin-angiotensin-aldosterone system. |
|
|
In heart failure, there is a compensatory increase in blood volume that serves to increase ?
|
In heart failure, there is a compensatory increase in blood volume that serves to increase ventricular preload and thereby enhance stroke volume by the Frank-Starling mechanism. Blood volume is augmented by a number of factors. Reduced renal perfusion results in decreased urine output and retention of fluid. Furthermore, a combination of reduced renal perfusion and sympathetic activation of the kidneys stimulates the release of renin, thereby activating the renin-angiotensin system. This, in turn, enhances aldosterone secretion. There is also an increase in circulating arginine vasopressin (antidiuretic hormone) that contributes to renal retention of water. The final outcome of humoral activation is an increase in renal reabsorption of sodium and water. The resultant increase in blood volume helps to maintain cardiac output; however, the increased volume can be deleterious because it raises venous pressures, which can lead to pulmonary and systemic edema. When edema occurs in the lungs, this can result in exertional dyspnea (shortness of breath during exertion). Therefore, most patients in heart failure are treated with diuretic drugs to reduce blood volume and venous pressures in order to reduce edema.
|
|
|
A 14 year old boy visits you, his GP, with symptoms of ongoing tiredness, headaches and breathlessness on exercise. You take a history, examine him and send blood for FBC (full blood count), iron studies and blood film.
His blood film is found to show hypochromic and microcytic indices. Which anaemia would typically present in this manner? B12 deficiency anaemia Iron deficiency anaemia Haemolytic anaemia Sickle cell anaemia Anaemia of chronic disease |
Iron deficiency anaemia typically produces a blood film which is both microcytic (small cells) and hypochromic (little colour).
In the western world, this may be produced by blood loss or less commonly dietary deficiency. James\' anaemia, however, is more likely to be due to insufficient iron in his diet as he is in his teenage years which typically have a high demand for iron due to growth spurts. |
|
|
You are an F2 doctor and see Mrs Alicia Farrell, a 58 year old university lecturer. She was referred by her GP and admitted to the medical unit. She presents with fever, malaise, weakness and bone pain. She tells you that she has had several urinary tract and lung infections in the past. The bone pain started five months ago and has been getting worse.
On examination you find that she has some petechiae (small pin-point haemorrhages on a body surface) in her lower extremities, tender sternum, a palpable spleen and enlarged lymph nodes throughout her body. You order blood tests and a blood film. The blood results show that she has anaemia, raised white cells with dysplastic changes (abnormal development) and low platelets. The blood film is below: What is the mostly diagnosis? Non-Hodgkins lymphoma Acute lymphoblastic leukaemia Acute myeloid leukaemia Hodgkins lymphoma Hairy cell leukaemia |
Acute myeloid leukaemia is caused by malignant transformation of a myeloid precursor cell. Myeloid cells normally differentiate into red cells, platelets, neutrophils, monocytes, eosinophils and basophils. This disease is uncommon in children and incidence increases with age. Symptoms are mainly a result of anaemia, neutropenia (decrease in the number of neutrophils) and thrombocytopenia (decrease in number of platelets). Patients normally present with recurrent infections (due to neutropenia), haemorrhage (due to thrombocytopenia), tiredness, malaise (due to anaemia) and bone pain (due to bone marrow infiltration by blast cells). They also present with splenomegaly and lymphadenopathy.
The blood film will show blast cells (immature cells) that contain primary granules, abundant basophilic cytoplasm and miss-placed nuclei. A small number of Auer rods, which are cylindrical stacks of dysplastic primary granules that fuse into thin needle-like structures, are also present in the blasts cells. |
|
|
You are an F2 doctor on a three month long oncology rotation. You see Mr Robert Flannigan, a 52 year old farmer, who has Non Hodgkins Lymphoma. He was initially asymptomatic, however enlarged mesenteric and hilar lymph nodes in the lungs were incidentally detected during a CT scan for another illness. This scan showed no other abnormalities other than these lymphadenopathies. Lymph node biopsy confirmed that he had Non Hodgkins Lymphoma. His Non Hodgkins lymphoma was then classed as stage III due to the spread above and below the diaphragm.
His recent blood test and the liver function test are all normal indicating no bone marrow and liver involvement. His serum lactate dehydrogenase (LDH) is not elevated. Based on the above evidence, what is the IPI (International prognostic index) score for Mr Flannigan? 5 4 3 2 1 |
The IPI predicts the probability of disease-free and overall survival based on the following factors, each of which scores one point on the IPI index. Patients in the higher risk groups have poor outcomes with conventional chemotherapy regimens.
Age greater than 60 years Stage of disease (Stage III or greater) Poor performance status (general health) More than one extra nodal site (sites other than lymph nodes) Presence of an elevated level of lactate dehydrogenase. Look back at your patient\'s history. Now try to work out his score and his survival. |
|
|
A 7 year old boy with known trisomy 21 (Down’s syndrome) presents to the paediatric outpatient department with a two month history of malaise, lethargy and exertional dyspnoea (shortness of breath) associated with several recent chest infections.
On examination he has several large bruises on his legs, generalised lymphadenopathy and hepatosplenomegaly. Blood was taken and the following results obtained: Haemoglobin (Hb) 9.6 g/dL (13.5-18.0 g/dl) White Cell Count (WCC) 12.4 x 10^9/l (4.0-11.0 x 10^9/l) Platelets 126 x 10^9/l (150-400 x 10^9/l) What is the most likely underlying diagnosis? Acute myelogenous leukaemia Chronic lymphocytic leukaemia Chronic myeloid leukaemia Acute lymphoblastic leukaemia Acute promyelocytic leukaemia |
Leukaemia is a cancer of the blood or bone marrow leading to abnormal proliferation of blood cells, usually white blood cells. Acute leukaemias are the proliferation of immature blood cells, whereas chronic leukaemia is characterised by proliferation of mature but otherwise abnormal blood cells. Both types lead to a displacement of normal cells within the bone marrow, leading to marrow failure or overflow of abnormal cells into the peripheral circulation, where they also disrupt function through displacement of normal cells. Whether a leukaemia is myelogenous or lymphocytic depends on the derivation of the abnormal cells:
Granulocytes (basophils, neutrophils and eosinophils) and monocytes in addition to red blood cells and platelets come from the myeloid stem cell, whereas B and T lymphocytes and Natural Killer cells come from lymphoid stem cells. Acute lymphoblastic leukaemia (ALL) is primarily a disease of childhood, and has a higher incidence in association with trisomy 21. Symptoms and signs include those of anaemia (tiredness, weakness, dyspnoea on exertion); repeated fever, infections and abscesses; bruising and/or bleeding especially of the oral mucosa, retina and lower limbs; and occasionally lymph node enlargement or hepatosplenomegaly. A full blood count (FBC) characteristically shows low haemoglobin with low platelet level and often raised white cell count, though this can be normal or decreased if there aren’t many circulating blast cells. This patient is young with a known chromosomal abnormality and an acute presentation of symptoms. |
|
|
Mrs Leyland a 60 year old supermarket cashier, attends to see you, her GP, with a six week history of dysphagia. She describes how she initially noticed a sensation of “food sticking” when eating a roast dinner around six weeks ago which she dismissed at the time. However, a week later the same thing happened again and has been getting more often ever since now occurring almost at every meal. She tells you only solid foods ‘get stuck’ and drinking fluids seems to help the sensation pass. She has no associated chest pain, dyspepsia, odynophagia (pain on swallowing), cough and is not nauseous. She has not regurgitated any foods and has noticed no gurgling or neck swelling. She tells you she has dropped a dress size over the last six weeks because of these swallowing problems.
She has no past medical history of note and is on no regular medications. She is a long term smoker, having smoked 15 cigarettes a day for 40 years and her alcohol intake is on average 18 units per day which you have been telling her is too high for some years. On examination, Mrs Leyland is not obviously anaemic, she has no lymphadenopathy and systems examination is normal. What is the most likely diagnosis? Achalasia Benign oesophageal stricture Pharyngeal pouch Diffuse oesophageal spasm Oesophageal cancer |
Oesophageal cancer does commonly cause dysphagia and weight loss and is the most likely diagnosis for Mrs Leyland given her history of rapidly progressing dysphagia to solids (indicating progressive obstruction) alongside her significant weight loss. Other symptoms can include retrosternal chest pain, coughing and with advanced disease patients can get a hoarse voice (due to involvement of the recurrent laryngeal nerve) and lymphadenopathy palpable in the supraclavicular fossa.
Mrs Leyland is a heavy smoker and drinks excess alcohol both of which are risk factors for developing oesophageal cancer. Other risk factors include poor diet, obesity and importantly long standing gastro-oesophageal reflux disease can lead to Barrett’s oesophagus which increases the risk over forty-fold. This is a condition whereby there has been metasplastic change of oesophageal squamous epithelium to more columnar-like cells so that the squamocolumnar junction appears to have migrated upwards on endoscopy. Oesophageal cancer needs to be investigated urgently with barium swallow, endoscopy to take a biopsy and endoscopic ultrasound to assess the depth of oesophageal wall invasion. |
|
|
You are a GP and see Frederick Turner a 65 year old local farmer. Frederick tells you he feels dreadfully low in energy, lethargic and has been having dizzy spells for the last 18 months. He has also been getting increasingly short of breath working on the farm. Until now he has attributed his symptoms to old age but last week he started to get a funny beating sensation in his chest which prompted him to see you today. He has not lost weight, his appetite has not changed and he says his mood is fine. All other systems history is unremarkable. Mr Turner has no past medical history of note and is on no regular medications. You measure his blood pressure which is healthy at 125/85.
You see from his notes that he has seen the nurse for cellulitis over the last two months and she has documented that he has reduced sensation bilaterally in both of his legs. Mr Turner tells you his legs have been very numb for over six months. You therefore decide to perform a full motor and sensory examination and note marked paraesthesia distally in both of Mr Turners legs. The rest of the neurological exam is unremarkable. You also perform GI, respiratory and cardiovascular examinations which too are unremarkable apart from you do notice Mr Turner is markedly pale. You decide therefore to send blood for a full blood count amongst your investigations. His haemoglobin returns as 7.8g/dL. Which of the following cause of anaemia is most likely? Anaemia of chronic disease Iron-deficiency anaemia Pernicious anaemia Thalassaemia Sickle cell anaemia |
Anaemia of chronic disease is associated with many illnesses such as infection, malignancy, rheumatoid arthritis and renal failure. Although anaemia of chronic disease is possible in this case it does not commonly result in such a severe anaemia and you would expect Mr. Turner to have systemic symptoms of the chronic disease. Which type of anaemia would also explain his neurological symptoms?
|
|
|
You are a GP. One of your patients and her husband have come to see you, the woman in considerable distress but the husband also clearly upset. Both she and her husband are oculocutaneous albinos, a type of albinism that affects both the skin and eyes.
She has recently given birth to their first child, who is clearly not an albino. Her brother-in-law has done some research on the internet and has told the family that, as albinism is an autosomal recessive disease, it is impossible for her husband to be the father. She is adamant that she has only had intercourse with her husband since they married over five years ago and that he must be the father of their child. Her husband says that he wants to believe her but, on the other hand, if the information on the internet is right then how can he believe her? Which of the following replies is the most likely explanation? The woman is lying – the only way the child can be non-albino is if someone other than her husband is the father of the child. The mutation causing albinism is unstable, the gene has spontaneously reverted to the normal sequence and so the child is phenotypically normal. Penetrance of the mutated gene for albinism is not 100% so the child could inherit mutated genes from both parents and still be phenotypically normal. More than one gene causes albinism – if the child has inherited one mutation from the father and a different mutation from the mother, the child would be phenotypically normal. Either the mother or the father is a genetic mosaic for albinism – some of their gametes will contain a normal copy of the affected gene and the child must have inherited one of these normal copies. |
Oculocutaneous albinism is a condition in which melanin is not produced either in the skin or in the retinas. There are several steps in the pathway involved in melanin production, each dependent on a different gene product. For example, a homozygous mutation either at 11q (tyrosinase) or at 15q (tyrosine transporter) will cause this form of albinism. If the mother has the mutation on 11q and the father on 15q, the child would inherit a normal copy of 15q from their mother and a normal copy of 11q from the father and so would be phenotypically normal.
|
|
|
You are a Foundation doctor on the cardiology team investigating a 49 year old man who has developed chest pain during exercise over the last 3 months. The pain is sometimes severe but goes within 10 minutes of stopping exercise.
He has no past medical history and is otherwise healthy, but has smoked 20 a day since the age of 16. You are concerned that this may be caused by myocardial ischaemia, and as part of his investigations organize an exercise ECG (treadmill test). Why is the heart particularly susceptible to ischaemia during exercise? Aortic dilatation during exercise partially occludes the coronary ostia, impairing coronary flow Cardiac muscle has low levels of myoglobin Circulating adrenaline prevents any increase in coronary blood flow Coronary arteries are already maximally dilated at rest Tachycardia decreases total diastolic time, impairing coronary perfusion |
The left ventricle is unusual in its blood supply in that coronary perfusion (its blood supply) is greater during diastole that systole. This results from extrinsic compression of the coronary vessels running through the myocardium as it contracts during systole.
As the heart rate increases, the main part of each cardiac cycle that is shortened is diastole, reducing the overall diastolic time for perfusion and thus limiting the ability of the coronary blood flow to increase during exercise. |
|
|
A 55 year old female presents to your GP surgery complaining of sudden attacks of excruciating, lightening-like jabs of facial pain. The pain can last for 15 minutes, and only affects one side of her face. She informs you that the pain is so intense that her husband has noticed her wincing.
Which nerve is most likely to be affected? Trigeminal Facial Vagus Vestibulocochlear Occulomotor |
The diagnosis is of trigeminal neuralgia, a condition affecting the sensory root of the trigeminal nerve. It usually affects the maxillary branch.
|
|
|
Left ventricular systolic dysfunction is commonly associated with
decreased end-diastolic pressure decreased end-systolic volume increased stroke work reduced ejection fraction |
reduced ejection fraction
|
|
|
Systolic dysfunction may result from which of the following cellular mechanisms?
reduced release of calcium by the sarcoplasmic reticulum increased activity of adenylyl cyclase increased activation of protein kinase A increased troponin C affinity for calcium |
reduced release of calcium by the sarcoplasmic reticulum
|
|
|
Stroke volume is reduced in left ventricular systolic dysfunction because ?
|
Stroke volume is reduced in left ventricular systolic dysfunction because muscle fiber shortening velocity is reduced at a given afterload and preload.
|
|
|
The increase in blood volume associated with chronic heart failure is caused by?
|
increased circulating aldosterone
|
|
|
Causes of Heart Failure ? (>10)
|
Causes of Heart Failure
•Myocardial infarction •Coronary artery disease •Chronic hypertension •Valve disease •Idiopathic cardiomyopathy •Viral or bacterial cardiomyopathy •Myocarditis •Pericarditis •Arrhythmias •Congenital heart disease •Diabetes •Thyroid disease •Pregnancy •Septic shock |
|
|
The number one cause of heart failure is ?
|
The number one cause of heart failure is coronary artery disease (CAD). CAD reduces coronary blood flow and oxygen delivery to the myocardium. This leads to myocardial hypoxia and impaired function. Another common cause of heart failure is myocardial infarction, which is the final and often fatal culmination of CAD. Infarcted tissue does not contribute to the generation of mechanical activity so overall cardiac performance is diminished. Furthermore, non-infarcted regions must compensate for the loss of function and this extra burden can precipitate changes that lead to failure. Valvular disease and congenital defects place increased demands upon the heart that can precipitate failure. Cardiomyopathies, of known origin (e.g., bacterial or viral) or idiopathic (unknown origin), can lead to failure. Myocarditis can have a similar effect. Arrhythmias such as severe bradycardia or tachycardia can also precipitate failure.
|
|
|
Preeclampsia is a disorder of widespread vascular endothelial malfunction and vasospasm that occurs after 20 weeks' gestation and can present as late as 4-6 weeks postpartum. It is clinically defined by ?
|
Preeclampsia is a disorder of widespread vascular endothelial malfunction and vasospasm that occurs after 20 weeks' gestation and can present as late as 4-6 weeks postpartum. It is clinically defined by hypertension and proteinuria, with or without pathologic edema.
|
|
|
Preeclampsia is mild in 75% of cases and severe in 25% of them.[7] In its extreme, the disease may lead to liver and renal failure, disseminated intravascular coagulopathy (DIC), and central nervous system (CNS) abnormalities. If preeclampsia-associated seizures develop, the disorder has developed into the condition called eclampsia.
Mild preeclampsia is defined as the presence of hypertension (BP ≥140/90 mm Hg) on 2 occasions, at least 6 hours apart, but without evidence of end-organ damage in the patient. Severe preeclampsia is defined as the presence of 1 of the following symptoms or signs in the presence of preeclampsia: ? |
Preeclampsia is mild in 75% of cases and severe in 25% of them.[7] In its extreme, the disease may lead to liver and renal failure, disseminated intravascular coagulopathy (DIC), and central nervous system (CNS) abnormalities. If preeclampsia-associated seizures develop, the disorder has developed into the condition called eclampsia.
Mild preeclampsia is defined as the presence of hypertension (BP ≥140/90 mm Hg) on 2 occasions, at least 6 hours apart, but without evidence of end-organ damage in the patient. Severe preeclampsia is defined as the presence of 1 of the following symptoms or signs in the presence of preeclampsia: • SBP of 160 mm Hg or higher or DBP of 110 mm Hg or higher on 2 occasions at least 6 hours apart • Proteinuria of more than 5 g in a 24-hour collection or more than 3+ on 2 random urine samples collected at least 4 hours apart • Pulmonary edema or cyanosis • Oliguria (< 400 mL in 24 h) • Persistent headaches • Epigastric pain and/or impaired liver function • Thrombocytopenia • Oligohydramnios, decreased fetal growth, or placental abruption |
|
|
Stage 1 cervical cancer - The smallest tumours of only a few millimetres (stage 1A1) are very unlikely to recur and have a cure rate of ?
|
Stage 1 cervical cancer means the cancer is only in the cervix. It is is now divided into 4 groups: stage 1A1, stage 1A2, stage 1B1 and stage 1B2, depending on the size of the cancer. The outcome or chance of being cured is better the earlier the cancer is detected. Smaller cancers have a better prognosis. The smallest tumours of only a few millimetres (stage 1A1) are very unlikely to recur and have a cure rate of 98 to 99%, if they are completely removed. For stage 1A2 cancers the cure rate is between 95 and 98%. For stage 1B1 cancers the cure rate is between 90 to 95%. A stage 1B2 cervical cancer, which may be larger than 4cm in diameter, still has a very good chance of cure. 8 out of 10 women (80%) with stage 1B2 cervical cancer will be cured.
|
|
|
Stage 1 cervical cancer - 1B1 cancers the cure rate is between %?
|
Stage 1 cervical cancer means the cancer is only in the cervix. It is is now divided into 4 groups: stage 1A1, stage 1A2, stage 1B1 and stage 1B2, depending on the size of the cancer. The outcome or chance of being cured is better the earlier the cancer is detected. Smaller cancers have a better prognosis. The smallest tumours of only a few millimetres (stage 1A1) are very unlikely to recur and have a cure rate of 98 to 99%, if they are completely removed. For stage 1A2 cancers the cure rate is between 95 and 98%. For stage 1B1 cancers the cure rate is between 90 to 95%. A stage 1B2 cervical cancer, which may be larger than 4cm in diameter, still has a very good chance of cure. 8 out of 10 women (80%) with stage 1B2 cervical cancer will be cured.
|
|
|
Stage 1 cervical cancer. For stage 1B1 cancers the cure rate is between % to %?
|
Stage 1 cervical cancer means the cancer is only in the cervix. It is is now divided into 4 groups: stage 1A1, stage 1A2, stage 1B1 and stage 1B2, depending on the size of the cancer. The outcome or chance of being cured is better the earlier the cancer is detected. Smaller cancers have a better prognosis. The smallest tumours of only a few millimetres (stage 1A1) are very unlikely to recur and have a cure rate of 98 to 99%, if they are completely removed. For stage 1A2 cancers the cure rate is between 95 and 98%. For stage 1B1 cancers the cure rate is between 90 to 95%. A stage 1B2 cervical cancer, which may be larger than 4cm in diameter, still has a very good chance of cure. 8 out of 10 women (80%) with stage 1B2 cervical cancer will be cured.
|
|
|
Stage 1 cervical cancer - A stage 1B2 cervical cancer, which may be larger than 4cm in diameter, still has a very good chance of cure. ?% with stage 1B2 cervical cancer will be cured?
|
Stage 1 cervical cancer means the cancer is only in the cervix. It is is now divided into 4 groups: stage 1A1, stage 1A2, stage 1B1 and stage 1B2, depending on the size of the cancer. The outcome or chance of being cured is better the earlier the cancer is detected. Smaller cancers have a better prognosis. The smallest tumours of only a few millimetres (stage 1A1) are very unlikely to recur and have a cure rate of 98 to 99%, if they are completely removed. For stage 1A2 cancers the cure rate is between 95 and 98%. For stage 1B1 cancers the cure rate is between 90 to 95%. A stage 1B2 cervical cancer, which may be larger than 4cm in diameter, still has a very good chance of cure. 8 out of 10 women (80%) with stage 1B2 cervical cancer will be cured.
|
|
|
groups: stages 2A and 2B. For all those women diagnosed with stage 2A cervical cancer, between 7 and 9 out of 10 (70 to 90%) what % will be alive 5 years later?
|
groups: stages 2A and 2B. For all those women diagnosed with stage 2A cervical cancer, between 7 and 9 out of 10 (70 to 90%) will be alive 5 years later.
For stage 2B the figures are slightly lower. Between 6 and 7 out of every 10 women (60 to 70%) will be alive 5 years after diagnosis. |
|
|
Cervical cancer - For stage 2B what % of women will be alive 5 years after diagnosis?
|
groups: stages 2A and 2B. For all those women diagnosed with stage 2A cervical cancer, between 7 and 9 out of 10 (70 to 90%) will be alive 5 years later.
For stage 2B the figures are slightly lower. Between 6 and 7 out of every 10 women (60 to 70%) will be alive 5 years after diagnosis. |
|
|
Stage 3 cervical cancer means the cancer has spread to the lower vagina or the side of the pelvis. What % of women will live at least five years after a diagnosis of stage 3 cervical cancer?
|
Stage 3 means the cancer has spread to the lower vagina or the side of the pelvis. As you might expect, the survival statistics fall with the more advanced stages of cervical cancer. Between 3 and 5 out of 10 women (30 to 50%) live at least five years after a diagnosis of stage 3 cervical cancer.
|
|
|
Stage 4 cervical cancer means the cancer has spread to distant organs or into the bladder or bowel. As it is the of women will live 5 years or longer with stage 4 cervical cancer?
|
Stage 4 means the cancer has spread to distant organs or into the bladder or bowel. As it is the most advanced stage, the survival statistics are lowest for stage 4 cervical cancers. 20 out of 100 women (20%) will live 5 years or longer with stage 4 cervical cancer. These are figures for all stage 4 cervical cancers. The figures will be slightly higher for women with stage 4A cancers and lower for those with stage 4B cancers.
|
|
|
Causes and Mechanisms of microcytic anaemia ?
|
Causes and Mechanisms of microcytic anaemia
Reduced iron availability deficiency Iron deficiency. Copper deficiency. Zinc poisoning. Reduced heme synthesis Congenital sideroblastic anaemia. Lead poisoning. Acquired sideroblastic anaemia, for example drugs or alcohol. Reduced globin production Thalassaemia syndromes. Other haemoglobinopathies. |
|
|
Menieres disease symptoms?
|
Common: vertigo (associated with nausea and vomiting), tinnitus, and a sense of pressure in the ear (aural fullness) - hearing loss is a mid to late symptom.
During severe attacks of vertigo, many people also suffer from diarrhoea, palpitations and sweating. |
|
|
Meniere's disease - Management ?
|
Meniere's disease - Management
When should I refer? To confirm the diagnosis of Meniere's disease — refer the person to an Ear, Nose, and Throat (ENT) consultant. If the person has symptoms and signs suggestive of hearing loss — refer for an audiology assessment, if not already carried by ENT services. People with severe symptoms may require hospital admission for intravenous (IV) labyrinthine sedatives and fluids to maintain hydration, and nutrition. Ideally involve the support of the multidisciplinary healthcare team (ENT, physiotherapist, hearing therapist, audiologist/clinical scientist, counsellor, or psychologist) early on so that people can benefit from their expertise. If Meniere's disease is poorly controlled or significantly impairs a person's quality of life, consider involvement of the multidisciplinary healthcare team if not previously done. |
|
|
What symptomatic treatment is recommended for an acute attack of Meniere's disease?
|
What symptomatic treatment is recommended for an acute attack of Meniere's disease?
To rapidly relieve (severe) nausea or vomiting associated with Meniere's disease, consider administration of buccal prochlorperazine, or a deep intramuscular injection of prochlorperazine. To help alleviate nausea, vomiting, and vertigo in other people with Meniere's disease, consider prescribing 7 days (14 days if required previously) of: Prochlorperazine, or An antihistamine (e.g. cinnarizine, cyclizine, or promethazine teoclate). Note: if symptoms are severe enough people may require hospital admission for intravenous (IV) labyrinthine sedatives and fluids to maintain hydration, and nutrition. |
|
|
What advice about driving is recommended for people with Meniere's disease?
|
What advice about driving is recommended for people with Meniere's disease?
Inform drivers with Meniere's disease–type symptoms that they are responsible for informing the Driver and Vehicle Licensing Agency (DVLA) of their condition. For group 1 entitlement (cars, motorcycles): Driving must cease on diagnosis. Driving will be permitted when satisfactory control of symptoms is achieved. The driving licence will be restored until a person is 70 years of age if they remain symptom free. For group 2 entitlement (lorries, buses): A licence will be refused or revoked if Meniere's disease is sudden or disabling. For detailed guidance, see the At-a-Glance Guide to the Current Medical Standards of Fitness to Drive. The underlying diagnosis should be considered and if it is likely to cause recurrent attacks of Meniere's disease, a person must be symptom free and controlled for at least 1 year before reapplication. |
|
|
How should I try and prevent recurrent attacks of Meniere's disease?
|
How should I try and prevent recurrent attacks of Meniere's disease?
Consider prescribing a trial of betahistine (initially 16 mg three times a day) to reduce the frequency and severity of attacks of hearing loss, tinnitus, and vertigo. The use of diuretics is not recommended for treating Meniere's disease in primary care. If betahistine does not provide the clinical benefit required, and people still have recurrent attacks of Meniere's disease despite its use, then referral to an Ear, Nose, and Throat (ENT) specialist is recommended for consideration of other possible interventions. Clarification / Additional information Evaluation of treatment to prevent attacks of Meniere's disease–type symptoms is difficult, owing to chronic course, fluctuating symptoms, variable remission periods, and significant placebo responses [James and Thorp, 2006]. Basis for recommendation The basis for this recommendation is that betahistine is licensed for treating hearing loss, tinnitus, and vertigo associated with Meniere's disease, and it is often used in clinical practice for this purpose [ABPI Medicines Compendium, 2006b]. Betahistine is a vasodilator and may work by reducing the pressure of fluid in the inner ear. Some randomized controlled trials have supported its use and others have have found betahistine to be no better than placebo. A Cochrane review has critically appraised the trials and concluded that there is insufficient evidence to say whether betahistine has any effect on Meniere's disease or not [James and Burton, 2001]. The manufacturers of betahistine recommend initially taking 16 mg three a day and advise that the duration of this initial dose should be based on the judgement of the clinician. CKS could find no further information on how long this initial dose should be taken for. |
|
|
A decrease in plasma bicarbonate can be caused by two mechanisms: ?
|
A decrease in plasma bicarbonate can be caused by two mechanisms:
A gain of strong acid A loss of base All causes of a metabolic acidosis must work by these mechanisms. The gain of strong acid may be endogenous (eg ketoacids from lipid metabolism) or exogenous (NH4Cl infusion). Bicarbonate loss may occur via the bowel (diarrhoea, small bowel fistulas) or via the kidneys (carbonic anhydrase inhibitors, renal tubular acidosis). |
|
|
Causes of Metabolic Acidosis (classified by Anion Gap)
A: High Anion-Gap Acidosis ? |
Causes of Metabolic Acidosis (classified by Anion Gap)
A: High Anion-Gap Acidosis 1. Ketoacidosis Diabetic ketoacidosis Alcoholic ketoacidosis Starvation ketoacidosis 2. Lactic Acidosis Type A Lactic acidosis (Impaired perfusion) Type B Lactic acidosis (Impaired carbohydrate metabolism) 3. Renal Failure Uraemic acidosis Acidosis with acute renal failure 4. Toxins Ethylene glycol Methanol Salicylates B : Normal Anion-Gap Acidosis (or Hyperchloraemic acidosis) 1. Renal Causes Renal tubular acidosis Carbonic anhydrase inhibitors 2. GIT Causes Severe diarrhoea Uretero-enterostomy or Obstructed ileal conduit Drainage of pancreatic or biliary secretions Small bowel fistula 3. Other Causes Recovery from ketoacidosis Addition of HCl, NH4Cl |
|
|
Causes of Metabolic Acidosis (classified by Anion Gap)
B : Normal Anion-Gap Acidosis (or Hyperchloraemic acidosis) ? |
Causes of Metabolic Acidosis (classified by Anion Gap)
A: High Anion-Gap Acidosis 1. Ketoacidosis Diabetic ketoacidosis Alcoholic ketoacidosis Starvation ketoacidosis 2. Lactic Acidosis Type A Lactic acidosis (Impaired perfusion) Type B Lactic acidosis (Impaired carbohydrate metabolism) 3. Renal Failure Uraemic acidosis Acidosis with acute renal failure 4. Toxins Ethylene glycol Methanol Salicylates B : Normal Anion-Gap Acidosis (or Hyperchloraemic acidosis) 1. Renal Causes Renal tubular acidosis Carbonic anhydrase inhibitors 2. GIT Causes Severe diarrhoea Uretero-enterostomy or Obstructed ileal conduit Drainage of pancreatic or biliary secretions Small bowel fistula 3. Other Causes Recovery from ketoacidosis Addition of HCl, NH4Cl |
|
|
AG is calculated from the following formula:?
|
AG is calculated from the following formula:
Anion gap = [Na+] - [Cl-] - [HCO3-] Reference range is 8 to 16 mmol/l. An alternative formula which includes K+ is sometimes used particularly by Nephrologists. In Renal Units, K+ can vary over a wider range and have more effect on the measured Anion Gap. This alternative formula is: AG = [Na+] + [K+] - [Cl-] - [HCO3-] The reference range is slightly higher with this alternative formula. The [K+] is low relative to the other three ions and it typically does not change much so omitting it from the equation doesn’t have much clinical significance. |
|
|
In ? metabolic acidosis (eg due HCl infusion), the infused Cl- replaces HCO3 and the anion gap remains normal. In ? acidosis, the lost bicarbonate is replaced by the acid anion which is not normally measured.
|
In an inorganic metabolic acidosis (eg due HCl infusion), the infused Cl- replaces HCO3 and the anion gap remains normal. In an organic acidosis, the lost bicarbonate is replaced by the acid anion which is not normally measured. This means that the AG is increased.
|
|
|
Causes of a high anion gap acidosis can be sorted out more specifically by using other investigations in addition to the history and examination of the patient. Investigations which may be very useful are:?
|
Causes of a high anion gap acidosis can be sorted out more specifically by using other investigations in addition to the history and examination of the patient. Investigations which may be very useful are:
Lactate Creatinine Plasma glucose Urine ketone test |
|
|
Hypoalbuminaemia does what to the anion gap ?
|
Hypoalbuminaemia causes a low anion gap
Albumin is the major unmeasured anion and contributes almost the whole of the value of the anion gap. Every one gram decrease in albumin will decrease anion gap by 2.5 to 3 mmoles. A normally high anion gap acidosis in a patient with hypoalbuminaemia may appear as a normal anion gap acidosis. This is particularly relevant in Intensive Care patients where lower albumin levels are common. A lactic acidosis in a hypoalbuminaemic ICU patient will commonly be associated with a normal anion gap. |
|
|
The liver is important in acid-base physiology and this is often overlooked. It is important because ?
|
The liver is important in acid-base physiology and this is often overlooked. It is important because it is a metabolically active organ which may be either a significant net producer or consumer of hydrogen ions. The amounts of acid involved may be very large. The acid-base roles of the liver may be considered under the following headings:
Carbon dioxide production from complete oxidation of substrates Metabolism of organic acid anions (such as lactate, ketones and amino acids) Metabolism of ammonium Production of plasma proteins (esp albumin) |
|
|
The acid-base roles of the liver may be considered under the following headings: ?
|
The liver is important in acid-base physiology and this is often overlooked. It is important because it is a metabolically active organ which may be either a significant net producer or consumer of hydrogen ions. The amounts of acid involved may be very large. The acid-base roles of the liver may be considered under the following headings:
Carbon dioxide production from complete oxidation of substrates Metabolism of organic acid anions (such as lactate, ketones and amino acids) Metabolism of ammonium Production of plasma proteins (esp albumin) |
|
|
Major Effects of a Metabolic Acidosis?
|
Major Effects of a Metabolic Acidosis
Respiratory Effects Hyperventilation ( Kussmaul respirations) - this is the compensatory response Shift of oxyhaemoglobin dissociation curve (ODC) to the right Decreased 2,3 DPG levels in red cells (shifting the ODC back to the left) Cardiovascular Effects Depression of myocardial contractility Sympathetic overactivity (incl tachycardia, vasoconstriction,decreased arrhythmia threshold) Resistance to the effects of catecholamines Peripheral arteriolar vasodilatation Venoconstriction of peripheral veins Vasoconstriction of pulmonary arteries Effects of hyperkalaemia on heart Other Effects Increased bone resorption (chronic acidosis only) Shift of K+ out of cells causing hyperkalaemia |
|
|
A metabolic acidosis is often strongly suspected because of the clinical presentation of the patient (eg diabetes, renal failure, severe diarrhoea). Three clues from a typical hospital automated biochemical profile are: ?
|
A metabolic acidosis is often strongly suspected because of the clinical presentation of the patient (eg diabetes, renal failure, severe diarrhoea). Three clues from a typical hospital automated biochemical profile are:
Low ‘bicarbonate’ (or low ‘total CO2’) High chloride High anion gap |
|
|
What is ‘total CO2’?
|
What is ‘total CO2’?
This is often reported as part of the laboratory’s automated biochemical profile on a venous blood sample. It represents the total concentration of all the species in the sample which can be converted to carbon dioxide gas. This is: Total CO2 = [HCO3] + [H2CO3] + [carbamino CO2] + [dissolved CO2] Apart from bicarbonate, all the other species are present in only small concentrations. The usefulness of the 'total CO2' is as an estimate of the arterial bicarbonate & which can be obtained without collecting an arterial sample. The value will usually be several mmols/liter higher than the actual arterial value due to the inclusion of carbamino & dissolved CO2 and because of the higher CO2 content of venous blood. Arterial blood gases are important for diagnosis but should always be interpreted in conjunction with the clinical details. In addition to arterial blood gases, some other investigations useful for indicating a metabolic acidosis and for differentiating between the various major causes are: Urine tests for glucose and ketones Electrolytes (incl chloride, anion gap, ‘bicarbonate’) Plasma glucose Urea and creatinine Lactate |
|
|
Causes of Respiratory Alkalosis?
|
Causes of Respiratory Alkalosis
1. Central Causes (direct action via respiratory centre) Head Injury Stroke Anxiety-hyperventilation syndrome (psychogenic) Other 'supra-tentorial' causes (pain, fear, stress, voluntary) Various drugs (eg analeptics, propanidid, salicylate intoxication) Various endogenous compounds (eg progesterone during pregnancy, cytokines during sepsis, toxins in patients with chronic liver disease) 2. Hypoxaemia (act via peripheral chemoreceptors) Respiratory stimulation via peripheral chemoreceptors 3. Pulmonary Causes (act via intrapulmonary receptors) Pulmonary Embolism Pneumonia Asthma Pulmonary oedema (all types) 4. Iatrogenic (act directly on ventilation) Excessive controlled ventilation |
|
|
'Causes' : Classification of Initiating Processes for Metabolic Alkalosis?
|
'Causes' : Classification of Initiating Processes for Metabolic Alkalosis
Gain of alkali in the ECF from an exogenous source (eg IV NaHCO3 infusion, citrate in transfused blood) from an endogenous source (eg metabolism of ketoanions to produce bicarbonate) Loss of H+ from ECF via kidneys (eg use of diuretics) via gut (eg vomiting, NG suction) |
|
|
'Causes' of clinically significant chronic metabolic alkalosis are usefully divided into 2 major groupings based on the major factor involved in the maintenance of the disorder: ?
|
'Causes' of clinically significant chronic metabolic alkalosis are usefully divided into 2 major groupings based on the major factor involved in the maintenance of the disorder:
The chloride depletion group The potassium depletion group Maintenance of the alkalosis requires a process which greatly impairs the kidney's ability to excrete bicarbonate and prevent the return of the elevated plasma level to normal. Chloride deficiency leads to a situation where the kidney reabsorbs more bicarbonate anion than usual because there is not sufficient chloride anion present. Reabsorption of an anion is necessary to maintain electroneutrality as Na+ & K+ are reabsorbed so the deficiency of chloride leads to a re-setting upwards of the maintained plasma bicarbonate level. Chloride and bicarbonate are the only anions present in appreciable quantities in extracellular fluid so a deficiency of one must lead to an increase in the other because of the strict requirement for macroscopic electroneutrality. |
|
|
Common Hybrid Classification of 'Causes' of Metabolic Alkalosis?
|
Common Hybrid Classification of 'Causes' of Metabolic Alkalosis
A: Addition of Base to ECF Milk-alkali syndrome Excessive NaHCO3 intake Recovery phase from organic acidosis (excess regeneration of HCO3) Massive blood transfusion (due metabolism of citrate) B: Chloride Depletion Loss of acidic gastric juice Diuretics Post-hypercapnia Excess faecal loss (eg villous adenoma) C: Potassium Depletion Primary hyperaldosteronism Cushing’s syndrome Secondary hyperaldosteronism Some drugs (eg carbenoxolone) Kaliuretic diuretics Excessive licorice intake (glycyrrhizic acid) Bartter's syndrome 1 Severe potassium depletion D: Other Disorders Laxative abuse 2,3,4 Severe hypoalbuminaemia 5 |
|
|
Bartter's syndrome?
|
Bartter's syndrome
This is a syndrome of increased renin and aldosterone levels due to hyperplasia of the juxtaglomerular apparatus 1,6. It is inherited as an autosomal recessive disorder. The increased aldosterone levels usually result in a metabolic alkalosis. The condition is usually found in children. Patients who present with hypokalaemic alkalosis of uncertain cause are often suspected of having this condition but other causes which may be denied by the patient should be considered eg surreptitious vomiting and/or use of diuretics for weight loss or psychological problems. These situations have been termed 'pseudo-Bartter's syndrome'. Rare genetic disorders such as Gitelmann's syndrome should also be considered. |
|
|
Metabolic Alkalosis Based on Urinary Chloride
Urine Cl- < 10 mmol/l - causes ? |
Metabolic Alkalosis Based on Urinary Chloride
Urine Cl- < 10 mmol/l Often associated with volume depletion (increased proximal tubular reabsorption of HCO3) Respond to saline infusion (replaces chloride and volume) Common causes: previous thiazide diuretic therapy, vomiting (90% of cases) Urine Cl- > 20 mmol/l Often associated with volume expansion and hypokalaemia Resistant to therapy with saline infusion Cause: Excess aldosterone, severe K+ deficiency Other causes: diuretic therapy (current), Bartter’s syndrome |
|
|
Metabolic Alkalosis Based on Urinary Chloride
Urine Cl- > 20 mmol/l - cause ? |
Metabolic Alkalosis Based on Urinary Chloride
Urine Cl- < 10 mmol/l Often associated with volume depletion (increased proximal tubular reabsorption of HCO3) Respond to saline infusion (replaces chloride and volume) Common causes: previous thiazide diuretic therapy, vomiting (90% of cases) Urine Cl- > 20 mmol/l Often associated with volume expansion and hypokalaemia Resistant to therapy with saline infusion Cause: Excess aldosterone, severe K+ deficiency Other causes: diuretic therapy (current), Bartter’s syndrome |
|
|
Metabolic alkalosis may be divided into two general groups based on ?
|
Metabolic alkalosis may be divided into two general groups based on the measured urinary chloride level.
In most cases the cause is obvious (eg vomiting, diuretic use) but if not then measurement of a spot urinary chloride can be useful. Two things to be aware of when interpreting the result: Recent diuretic use can acutely elevate the urinary chloride level but as the diuretic effect passes the urinary chloride level will fall to low levels. So seek information on the timing of diuretic use when assessing the result. (This variability in urine chloride levels has been used as an indicator of surreptious diuretic use). A 'spot' urine chloride may be misleading if bladder urine contains a mixture of urine from during and after diuretic effect. A high urinary chloride in association with hypokalaemia suggests mineralocorticoid excess (provided that recent thiazide use has been excluded). If the clinical information is not sufficient to make a diagnosis the term 'idiopathic metabolic alkalosis' is sometimes used. The urinary chloride/creatinine ratio may occasionally be useful as it is elevated if there is an extra-renal cause of alkalosis. Metabolic Alkalosis Based on Urinary Chloride Urine Cl- < 10 mmol/l Often associated with volume depletion (increased proximal tubular reabsorption of HCO3) Respond to saline infusion (replaces chloride and volume) Common causes: previous thiazide diuretic therapy, vomiting (90% of cases) Urine Cl- > 20 mmol/l Often associated with volume expansion and hypokalaemia Resistant to therapy with saline infusion Cause: Excess aldosterone, severe K+ deficiency Other causes: diuretic therapy (current), Bartter’s syndrome |
|
|
A 16-year-old male was involved in a gang fight and was stabbed in the popiteal fossa. The patient developed a calcaneovalgus deformity of the foot. He is unable to tip toe or invert his foot. He has loss of sensation over his sole.
|
The tibial nerve originates from the sciatic nerve and descends through the popiteal fossa through the posterior compartment of the leg and divides into medial and lateral plantar nerves. It supplies the posterior leg muscles and knee.
|
|
|
The tibial nerve originates from the sciatic nerve and descends through the popiteal fossa through the posterior compartment of the leg and divides into medial and lateral plantar nerves. It supplies ?
|
The tibial nerve originates from the sciatic nerve and descends through the popiteal fossa through the posterior compartment of the leg and divides into medial and lateral plantar nerves. It supplies the posterior leg muscles and knee.
|
|
|
A 65-year-old female with osteoporosis undergoes a total hip replacement. After the operation she is unable to dorsiflex and plantarflex her foot, she also has loss of sensation over the lateral aspect of her leg.
Please select an optionCommon peroneal nerveDeep peroneal nerveFemoral nerveGenitofemoral nerveIlioinguinal nerveSaphenous nerveSciatic nerveSuperficial peroneal nerveSural nerveTibial nerve |
The correct answer is Sciatic nerve
The sciatic nerve originates from the sacral plexus and enters the gluteal region and descends along the posterior aspect of thigh. It divides into the tibial and common peroneal nerve. It supplies the hamstrings and supplies the hip and knee. |
|
|
A 28-year-old male suffered a motorcycle accident and dislocated his knee. He is unable to dorsiflex his foot and toes, and has foot-drop and a high stepping gait. There is loss of sensation over dorsum of foot.
|
The correct answer is Common peroneal nerve
The common peroneal nerve originates from the sciatic nerve and divides into the superficial and deep peroneal nerve. This nerve is the most commonly injured as it winds superficially around the neck of fibula, which can be injured if the neck of fibula fractures. |
|
|
Following injury, a 20-year-old male presents with weakness of elbow flexion and supination. There is loss of sensation over lateral surface of forearm.
|
The musculocutaneous nerve supplies the coracobrachialis, biceps and brachialis muscle. It also supplies sensation to the lateral surface of forearm by the lateral antebrachial cutaneous nerve.
|
|
|
A 52-year-old diabetic male presents with weakness of elbow extension and he has an inability to extend wrist resulting in wrist-drop.
|
The radial nerve supplies the triceps, brachioradialis, supinator and extensor muscles of wrist and digits.
|
|
|
A 42-year-old male with polyarteritis nodosa presents with weakness of the thenar muscles and adjacent two lumbricals. He has an inability to oppose the thumb with little finger. You also note a loss of sensation over thumb and index, middle and lateral half of ring finger.
|
The median nerve supplies the lateral two lumbricals, opponens pollicis, abductor pollicis and flexor pollicis brevis.
|
|
|
A 42-year-old male presents following injury with a loss of sensation over the medial one and half fingers. You also note weakness of adduction of the thumb as well as small muscles of hand and the hand assumes a claw appearance.
|
The ulnar muscle supplies all small muscles of the hand except for LOAF which are supplied by the median nerve.
|
|
|
You see a 16-year-old male who following a birth injury has a right arm weakness with the assumption of a waiter's tip position.
|
The superior brachial plexus may become injured during motorcycle accidents or due to excess stretching on a newborn's arm.
|
|
|
The muscles of the hand supplied by the median nerve can be remembered using the mnemonic, "LOAF" ?
|
The muscles of the hand supplied by the median nerve can be remembered using the mnemonic, "LOAF" for Lumbricals 1 & 2, Opponens pollicis, Abductor pollicis brevis and Flexor pollicis brevis. (NB: OAF are the thenar eminence)[
|
|
|
In the hand, the median nerve supplies motor innervation to the 1st and 2nd lumbrical muscles. It also supplies the muscles of the thenar eminence by a recurrent thenar branch. The rest of the intrinsic muscles of the hand are supplied by which nerve?
|
In the hand, the median nerve supplies motor innervation to the 1st and 2nd lumbrical muscles. It also supplies the muscles of the thenar eminence by a recurrent thenar branch. The rest of the intrinsic muscles of the hand are supplied by the ulnar nerve.
|
|
|
Following a stroke, a 72-year-old male has pure expressive aphasia.
Please select an optionFrontal lobe, bilateralFrontal lobe left sideFrontal lobe right sideTemporo-parietal region left sideTemporo-parietal region right sideTemporal lobe left sideTemporal lobe right sideParietal lobe, left sideParietal lobe, right sideOccipital lobe, bilaterallyOccipital lobe, left sideOccipital lobe, right side |
The correct answer is Frontal lobe left side
|
|
|
A 73-year-old female presents with sudden onset receptive aphasis, acalculia, agraphia and has a right homonymous field defect.
Please select an optionFrontal lobe, bilateralFrontal lobe left sideFrontal lobe right sideTemporo-parietal region left sideTemporo-parietal region right sideTemporal lobe left sideTemporal lobe right sideParietal lobe, left sideParietal lobe, right sideOccipital lobe, bilaterallyOccipital lobe, left sideOccipital lobe, right side |
The correct answer is Temporo-parietal region left side
|
|
|
A 82-year-old male presents with increasing confusion, an inability to recognise familiar faces and has a left homonymous field defect
Please select an optionFrontal lobe, bilateralFrontal lobe left sideFrontal lobe right sideTemporo-parietal region left sideTemporo-parietal region right sideTemporal lobe left sideTemporal lobe right sideParietal lobe, left sideParietal lobe, right sideOccipital lobe, bilaterallyOccipital lobe, left sideOccipital lobe, right side |
The correct answer is Temporal lobe right side
|
|
|
A lesion to the left side of the frontal lobe causes reduced fluency of speech with comprehension preserved, this due to what area being damaged ?
|
A lesion to the left side of the frontal lobe causes reduced fluency of speech with comprehension preserved, this due to Broca's area being damaged. Patients who recover report that they know what they wanted to say but could not get the words out.
|
|
|
A lesion to the left ? (Wernicke's area) causes fluency of speech to be preserved but the words come out incorrectly ?
|
A lesion to the left temporo-parietal (Wernicke's area) causes fluency of speech to be preserved but the words come out incorrectly. Patients who recover report that they did not understand their or anyone else's speech.
|
|
|
Tip of the shoulder
Please select an optionC4C5C6C7C8T1T2T3T4 |
The correct answer is C4
|
|
|
Knee
Please select an optionL1L2L3L4L5S1S2S3S4S5 |
L3 knee!
|
|
|
Fifth digit of the foot
Please select an optionL1L2L3L4L5S1S2S3S4S5 |
The correct answer is S1
|
|
|
Anus
Please select an optionL1L2L3L4L5S1S2S3S4S5 |
The correct answer is S5
|
|
|
An acute spastic paraparesis is a neurological emergency, suggesting the compression of the spinal cord at levels ranging typically from the thoracic to lumbar vertebrae.
It is caused by vascular disorders, osteoporotic collapse of the vertebrae or, more commonly, metastatic infiltration of the spine. Typically, one sees ? |
An acute spastic paraparesis is a neurological emergency, suggesting the compression of the spinal cord at levels ranging typically from the thoracic to lumbar vertebrae.
It is caused by vascular disorders, osteoporotic collapse of the vertebrae or, more commonly, metastatic infiltration of the spine. Typically, one sees upper motor neurone features - increased tone in the lower limbs, up-going plantars (extensor), exaggerated reflexes, weakness and a sensory level, that is, sensation is typically lost to the level of the lesion. The sensory loss is often related to light touch and proprioception, but depending on the extent of infiltration/compression, pain will also be lost. Urinary retention is also a feature. |
|
|
A 65-year-old male has a three month history of falls, weakness and difficulty walking. He has a spastic gait with exaggerated reflexes and reduced light sensation to the umbilicus.
Please select an optionAlzheimer’s diseaseCerebellar infarctCharcot Marie ToothCord compressionGuillian-Barre syndromeMeniere’s diseaseMultiple sclerosisNormal pressure hydrocephalusOsteomalaciaParkinson’s disease |
Cord compression
|
|
|
A 55-year-old male presents acutely with vomiting and vertigo. On examination he is markedly unsteady, falls to the left and has nystagmus of the eyes to the left.
Please select an optionAlzheimer’s diseaseCerebellar infarctCharcot Marie ToothCord compressionGuillian-Barre syndromeMeniere’s diseaseMultiple sclerosisNormal pressure hydrocephalusOsteomalaciaParkinson’s disease |
Cerebellar infarct
|
|
|
Parietal lobe tumour
Please select an optionBitemporal hemianopiaCentral scotomaCortical blindnessEnlarged blind spotHomonymous hemianopiaInferior homonymous quadrantanopiaSuperior homonymous quadrantanopiaTunnel visionUniocular blindness |
The correct answer is Inferior homonymous quadrantanopia
|
|
|
Central retinal artery occlusion
Please select an optionBitemporal hemianopiaCentral scotomaCortical blindnessEnlarged blind spotHomonymous hemianopiaInferior homonymous quadrantanopiaSuperior homonymous quadrantanopiaTunnel visionUniocular blindness |
The correct answer is Uniocular blindness
|
|
|
A parietal lobe tumour is associated with ?
|
A parietal lobe tumour is associated with disturbed sensation including localisation of touch and disturbed two-point discrimination. The typical associated visual field defect is a lower homonymous quadrantanopia and it affects the upper fibres of the optic radiation. Light sensation is finally received in the occipital cortex.
|
|
|
An 83-year-old woman presents to the Emergency Department after a fall. x Ray confirms a fractured distal radius. On examination, there is loss of sensation over the thumb, index and middle fingers.
Please select an optionAnterior interrosseous nerveLong thoracic nerveMedian nerveMusculocutaneous nervePalmar cutaneous branch of median nervePosterior interosseous nerveRadial nerveSuprascapular nerveUlnar nerve |
The correct answer is Palmar cutaneous branch of median nerve
Median nerve injury at the wrist causes sensory loss over the thumb index finger middle finger occasionally ring finger (lateral half). Motor loss includes all thenar muscles except adductor pollicis (supplied by ulnar nerve) and lateral two lumbricals. |
|
|
the following are found in a IIIrd nerve palsy?
|
The pupil is dilated.
Ptosis There is a divergent squint with the affected eye deviated 'down and out'. The third cranial nerve (oculomotor) innervates the muscles in the eye that are responsible for the movement of the eye upwards, downwards and to the mid line. It does not innervate the muscle responsible for the movement of the eye outwards (lateral rectus innervated by the abducens nerve), nor the superior oblique muscle responsible for the eye looking down. The third nerve also innervates the pupil. Thus, the appearance of the eye is that it looks downwards and outwards (because of the unopposed pull of the functioning muscles) and the pupil is dilated. Third nerve palsy can be due to a diabetic neuropathy, vasculitis or a compression of the third nerve by an intracranial tumour or aneurysm (anterior and posterior communicating artery aneurysm). |
|
|
Horner's syndrome - ptosis, miosis and mydriasis (dryer right side due to reduced sweating) and is due to sympathetic de-innervation at any point in the course of the sympathetic supply to the eye.
It is associated with? |
Horner's syndrome - ptosis, miosis and mydriasis (dryer right side due to reduced sweating) and is due to sympathetic de-innervation at any point in the course of the sympathetic supply to the eye.
It is associated with Apical tumours of the lung (Pancoast tumours) Cervical rib Goitre Syringomyelia and Lateral medullary syndrome (brainstem stroke). |
|
|
Anterior aspect of thigh and knee
Please select an optionL1L2L3L4L5S1S2S3S4 |
L3
|
|
|
Medial aspect and dorsum of the foot together with the great toe
Please select an optionL1L2L3L4L5S1S2S3S4 |
L5
|
|
|
Posterior aspect of the calf and knee
Please select an optionL1L2L3L4L5S1S2S3S4 |
S2
|
|
|
Sole and lateral aspect of foot
Please select an optionL1L2L3L4L5S1S2S3S4 |
L1
|
|
|
Please select an optionFasciculus cuneatusFasciculus gracilisLateral corticospinal tractLateral spinothalamic tractLissauer's tractMedial longitudinal fasciculusSpinocuneocerebellar tractTrigeminal tractVentral (anterior) spinocerebellar tract
|
Fasciculus gracilis
|
|
|
Temperature and pain sensation from the contralateral limb.
Please select an optionFasciculus cuneatusFasciculus gracilisLateral corticospinal tractLateral spinothalamic tractLissauer's tractMedial longitudinal fasciculusSpinocuneocerebellar tractTrigeminal tractVentral (anterior) spinocerebellar tract |
The correct answer is Lateral spinothalamic tract
|
|
|
Temperature and pain sensation from the ipsilateral limb carried up spinal cord segments before decussation.
Please select an optionFasciculus cuneatusFasciculus gracilisLateral corticospinal tractLateral spinothalamic tractLissauer's tractMedial longitudinal fasciculusSpinocuneocerebellar tractTrigeminal tractVentral (anterior) spinocerebellar tract |
The correct answer is Lissauer's tract
|
|
|
Upper limb vibration sense.
Please select an optionFasciculus cuneatusFasciculus gracilisLateral corticospinal tractLateral spinothalamic tractLissauer's tractMedial longitudinal fasciculusSpinocuneocerebellar tractTrigeminal tractVentral (anterior) spinocerebellar tract |
Fasciculus cuneatus
|
|
|
High step gait
Please select an optionBilateral upper motor neurone damageCerebellar damageExtrapyramidal diseaseHip dysplasiaOsteoarthritisPeripheral motor neuropathyPeripheral sensory neuropathyProximal myopathy |
Peripheral motor neuropathy
|
|
|
Shuffling gait
Please select an optionBilateral upper motor neurone damageCerebellar damageExtrapyramidal diseaseHip dysplasiaOsteoarthritisPeripheral motor neuropathyPeripheral sensory neuropathyProximal myopathy |
Extrapyramidal disease
|
|
|
Waddling gait
Please select an optionBilateral upper motor neurone damageCerebellar damageExtrapyramidal diseaseHip dysplasiaOsteoarthritisPeripheral motor neuropathyPeripheral sensory neuropathyProximal myopathy |
Proximal neuropathy
|
|
|
Internal capsular infarction
Please select an optionBitemporal hemianopiaCentral scotomaCortical blindnessEnlarged blind spotHomonymous hemianopiaInferior homonymous quadrantanopiaSuperior homonymous quadrantanopiaTunnel visionUniocular blindness |
Internal capsular infarct is the commonest cerebral infarct associated with contralateral hemiparesis and homonymous hemianopia where there is optic radiation damage causing loss of the ipsilateral nasal field and contralateral temporal field.
|
|
|
Temporal lobe tumour
Please select an optionBitemporal hemianopiaCentral scotomaCortical blindnessEnlarged blind spotHomonymous hemianopiaInferior homonymous quadrantanopiaSuperior homonymous quadrantanopiaTunnel visionUniocular blindness |
Temporal lobe tumours result in receptive dysphasia and upper quadrantic field defects.
|
|
|
Taste to the posterior thrid of the tongue
Please select an optionAbducens nerveAccessory nerveFacial nerveGlossopharyngeal nerveHypoglossal nerveOculomotor nerveOlfactory nerveOptic nerveTrigeminal nerveTrochlear nerveVagus nerveVestibulo-cochlear nerve |
The correct answer is Glossopharyngeal nerve
|
|
|
Taste to the anterior two-thirds of the tongue
Please select an optionAbducens nerveAccessory nerveFacial nerveGlossopharyngeal nerveHypoglossal nerveOculomotor nerveOlfactory nerveOptic nerveTrigeminal nerveTrochlear nerveVagus nerveVestibulo-cochlear nerve |
The correct answer is Facial nerve
|
|
|
Complications of lung cancer ?
|
Complications: Lung cancer
Wheezing - by narrowing the airway Atelectasis - by blocking an airway leading to the collapse of the part of the lung that the airway supplies Shortness of breath Pneumonia, which may result in coughing, fever, and chest pain If the tumor grows into the chest wall, it may produce persistent, unrelenting chest pain. Pleural Effusion - fluid containing cancerous cells can accumulate in the space between the lung and the chest wall RVH, heart failure, cor pulmonale Horner's - ptosis, small pupil, sunken eye, and reduced perspiration on one side of the face Pancoast' tumor - cancers at the top of the lung - may grow into the nerves that supply the arm, making the arm painful, numb, and weak When the tumor grows into nerves in the center of the chest, the nerve to the voice box may become damaged, making the voice hoarse. Lung cancer may grow into or near the esophagus, leading to difficulty swallowing or pain with swallowing. - Lung cancer may grow into the heart or in the midchest (mediastinal) region, causing abnormal heart rhythms, blockage of blood flow through the heart, or pericardial effusion - superior vena cava syndrome - obstruction of the superior vena cava causes blood to back up in other veins of the upper body. The veins in the chest wall enlarge. The face, neck, and upper chest wall—including the breasts—can swell, causing pain. The condition can also produce shortness of breath, headache, distorted vision, dizziness, and drowsiness. These symptoms usually worsen when the person bends forward or lies down. Lung cancer may also spread through the bloodstream to other parts of the body, most commonly the liver, brain, adrenal glands, spinal cord, or bones. Symptoms—such as headache, confusion, seizures, and bone pain—may develop before any lung problems become evident, making an early diagnosis more complicated. Paraneoplastic syndromes - consist of effects that are caused by cancer but occur far from the cancer itself, such as in nerves and muscles. These syndromes are not related to the size or location of the lung cancer and do not necessarily indicate that the cancer has spread outside the chest. These syndromes are caused by substances secreted by the cancer (such as hormones, cytokines, and various other proteins). |

