![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
43 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
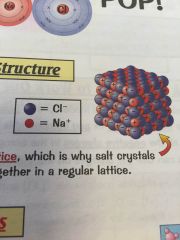
Whats the structure of an ionic compound?
|
•ionic compounds always have giant ionic
•the ions form a closely packed regular lattice arrangement •there are very strong electrostatic forces of attraction between oppositely charged ions |
|
|
|
How to show the electronic structure of simple ions with diagrams
|

|
|
|
|
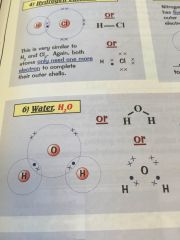
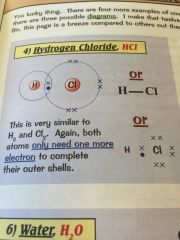
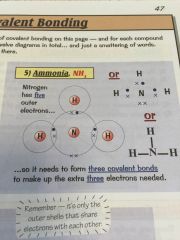
Whats covalent bonding?
|

•sharing electrons -in their outer shell-
•to make it that both atoms have a full outer shell |

|
|
Examples of covalent bonds
|
|
|
|
|
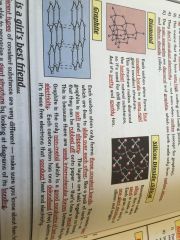
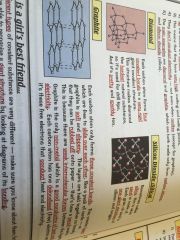
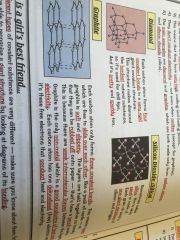
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
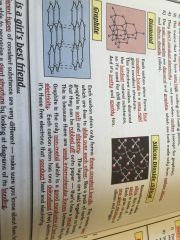
Examples;
|
|
|
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
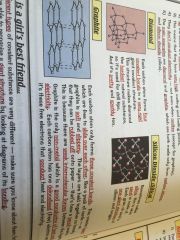
Examples;
|
|
|
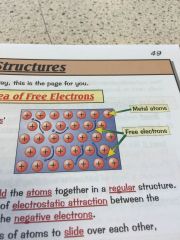
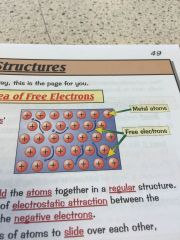
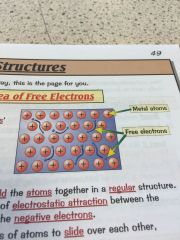
Whats the sea of free electrons and why does it make metals good conductors?
|
•metals consits of a giant structure
•metallic bonds have free electrons which produce the properties of metals • the delocalised -free- electrons come from the outer shell of every metal atom •the electrons are free to move through the whole structure meaning metals are good conductors •they also hold the atoms together in a regular structure meaning they can be bent and shaped. |
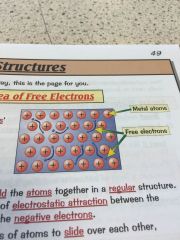
|
|
|
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
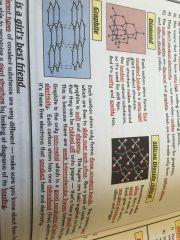
Examples;
|
|
|
Whats the sea of free electrons and why does it make metals good conductors?
|
•metals consits of a giant structure
•metallic bonds have free electrons which produce the properties of metals • the delocalised -free- electrons come from the outer shell of every metal atom •the electrons are free to move through the whole structure meaning metals are good conductors •they also hold the atoms together in a regular structure meaning they can be bent and shaped. |
|
|
|
Smart materials- nithinol
|
•smart materials behave differently
•eg, nitinol - a shape memory alloy •its half nickel, half titanium and you can bend and twist it like rubber •if you heat it it goes back to its remembered shape |
|
|
|
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
Examples;
|
|
|
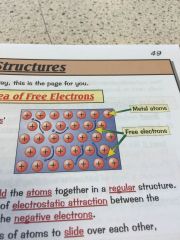
Whats the sea of free electrons and why does it make metals good conductors?
|
•metals consits of a giant structure
•metallic bonds have free electrons which produce the properties of metals • the delocalised -free- electrons come from the outer shell of every metal atom •the electrons are free to move through the whole structure meaning metals are good conductors •they also hold the atoms together in a regular structure meaning they can be bent and shaped. |
|
|
|
Smart materials- nithinol
|
•smart materials behave differently
•eg, nitinol - a shape memory alloy •its half nickel, half titanium and you can bend and twist it like rubber •if you heat it it goes back to its remembered shape |
|
|
|
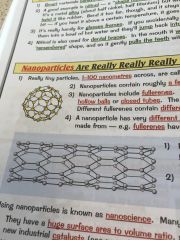
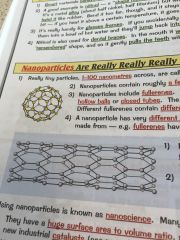
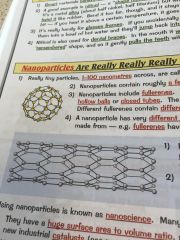
What are nanoparticles?
|
•really tiny particles, 1-100 nanometers
•contains roughly a few hundred atoms •they include fullerenes-these are molecules of carbon, arranged in hexagonal rings •can be joined together to form nanotubes, the covalent bonds make them very strong |
|
|
|
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
Examples;
|
|
|
Whats the sea of free electrons and why does it make metals good conductors?
|
•metals consits of a giant structure
•metallic bonds have free electrons which produce the properties of metals • the delocalised -free- electrons come from the outer shell of every metal atom •the electrons are free to move through the whole structure meaning metals are good conductors •they also hold the atoms together in a regular structure meaning they can be bent and shaped. |
|
|
|
Smart materials- nithinol
|
•smart materials behave differently
•eg, nitinol - a shape memory alloy •its half nickel, half titanium and you can bend and twist it like rubber •if you heat it it goes back to its remembered shape |
|
|
|
What are nanoparticles?
|
•really tiny particles, 1-100 nanometers
•contains roughly a few hundred atoms •they include fullerenes-these are molecules of carbon, arranged in hexagonal rings •can be joined together to form nanotubes, the covalent bonds make them very strong |
|
|
|
How do the forces between molecules determine the properties of plastics?
|

|

|
|
|
Macromolecules
|
•similar to giant lattices except there are no charged ions
•bonded by strong covalent bonds •hight melting and boiling points |
Examples;
|
|
|
Whats the sea of free electrons and why does it make metals good conductors?
|
•metals consits of a giant structure
•metallic bonds have free electrons which produce the properties of metals • the delocalised -free- electrons come from the outer shell of every metal atom •the electrons are free to move through the whole structure meaning metals are good conductors •they also hold the atoms together in a regular structure meaning they can be bent and shaped. |
|
|
|
Smart materials- nithinol
|
•smart materials behave differently
•eg, nitinol - a shape memory alloy •its half nickel, half titanium and you can bend and twist it like rubber •if you heat it it goes back to its remembered shape |
|
|
|
What are nanoparticles?
|
•really tiny particles, 1-100 nanometers
•contains roughly a few hundred atoms •they include fullerenes-these are molecules of carbon, arranged in hexagonal rings •can be joined together to form nanotubes, the covalent bonds make them very strong |
|
|
|
How do the forces between molecules determine the properties of plastics?
|

|

|
|
|
How making polymers affects its properties?
|
•two types of polythene
•low density-heating ethene to about 200 degrees under high pressure, its flexible and used for bags and bottles •high density polythene is made at lower temperatures and pressure-with catalyst- its more rigid and is used for fish tanks and drainpipes |
|
|
|
Whats relative atomic mass and where would you find it?
|

•its the mass of the atom
|
|
|
|
Whats relative atomic mass and where would you find it?
|

•its the mass of the atom
|
|
|
|
Whats relative formula mass?
|
•if you have a compound like MgCl2 it have a relative formula mass, which are all the relative atomic masses added together
•example: 24 + (35.5 x 2) =95 |

|
|
|
Whats relative atomic mass and where would you find it?
|

•its the mass of the atom
|
|
|
|
Whats relative formula mass?
|
•if you have a compound like MgCl2 it have a relative formula mass, which are all the relative atomic masses added together
•example: 24 + (35.5 x 2) =95 |

|
|
|
One mole
|

|
|
|
|
How do you calculate percentage mass of an element in a compound
|
%mass=(atomic mass x no. of atoms/formula mass) x100
|

Example; find the %mass of sodium in na2co3
|
|
|
How do you calculate percentage mass of an element in a compound
|
%mass=(atomic mass x no. of atoms/formula mass) x100
|

Example; find the %mass of sodium in na2co3
|
|
|
How to find the empirical formula?
|
•empirical formula is the only way of finding the formula for that compound
•list all the elements in the compound •underneath write their experimental masses/ % •divide each mass by the atomic mass •turn the numbers into a ratio |

Example
|
|
|
How do you calculate percentage mass of an element in a compound
|
%mass=(atomic mass x no. of atoms/formula mass) x100
|

Example; find the %mass of sodium in na2co3
|
|
|
How to find the empirical formula?
|
•empirical formula is the only way of finding the formula for that compound
•list all the elements in the compound •underneath write their experimental masses/ % •divide each mass by the atomic mass •turn the numbers into a ratio |

Example
|
|
|
Whats %yield and how to work it out?
|
• the amount of product you het is known as yield
•predicted yield can be calculated from the balanced reaction • always between 0-100% •0% means no reactants were converted into product. |

|
|
|
How do you calculate percentage mass of an element in a compound
|
%mass=(atomic mass x no. of atoms/formula mass) x100
|

Example; find the %mass of sodium in na2co3
|
|
|
How to find the empirical formula?
|
•empirical formula is the only way of finding the formula for that compound
•list all the elements in the compound •underneath write their experimental masses/ % •divide each mass by the atomic mass •turn the numbers into a ratio |

Example
|
|
|
Whats %yield and how to work it out?
|
• the amount of product you het is known as yield
•predicted yield can be calculated from the balanced reaction • always between 0-100% •0% means no reactants were converted into product. |

|
|
|
Reversable reaction
|

|
|
|
|
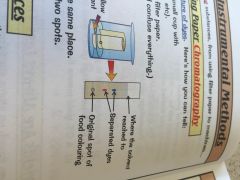
How to separate artificial colours using chromatography?
|
•put spots of the coloured solution on a pencil baseline on filter paper
• roll the sheet and put it in a beaker with some solvent-keep baseline above solvent- •solvent seeps up the paper, different dyes form spots in different places |
|
|
|
How is gas chromatography used to identify substances?
|
•gas is used to carry substances through a column
• substances travel at different speeds so they separate • the time taken to reach the detector is called the retention time |

|

