![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
221 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What are some (theoretical) advantages/disadvantages of CVVHD compared to IHD
|
1. Less intradialytic hypotension (better hemodynamic stability)
2. More constant volume control and plasma solute concentration. 3. Higher weekly solute clearance (unless IHD administered daily). 4. Possible slight mortality advantage, but likely dose of dialysis delivered is more important than the mode. Data are controversial. Ref: SCCM Adult Multiprofessional Critical Care Review pg. 263. - Kt/V urea (a dimensionless index of the dialysis dose in which K is the urea clearance of the dialyzer, t is the duration of dialysis, and V is the volume of distribution of urea) of 1.2 to 1.4 per session or average per week. |
|
|
|
What is the formula for fractional excretion of Na+?How is it used clinically?
|
FeNa = [(UNa x PCr)/(UCr x PNa)] x 100
To accurately interpret FENa, patients should not have recently received diuretics. FENa is greater than 1% and usually greater than 3% with acute tubular necrosis and severe obstruction of the urinary drainage of both kidneys. It is generally less than 1% in patients with acute glomerulonephritis, hepatorenal syndrome, and states of prerenal azotemia such as congestive heart failure or dehydration. FENa may also be less than 1% with acute partial urinary tract obstruction. For patients on diuretics, can use fractional excretion of urea or lithium. -- Stein JH, ed. Internal Medicine, 4th edition. Mosby-Year Book. 1994. |
|
|
|
What are the clinical criteria for diagnosis of brain death prior to apnea testing?
|
Preconditions:
1) Cause of coma is known 2) Cause is adequate to explain irreversible cessation of whole brain function. 3) irreversible brain catastrophe which involves both the cerebral hemispheres and the brain stem. 4) core temperature > 34 C 5) no evidence of intoxication, poisoning, or the use of paralytics, anesthetics or sedatives 6) no confounding medical conditions such as severe endocrine, electrolyte or acid-base disturbances. 7) Absence of spontaneous movement and brain stem reflexes (pupillary response, oculo-cephalic and oculo-caloric reflexes, gag). |
|
|
|
What are the criteria to satisfy apnea testing?
|
1. Core temp. >32.2, preferably >36.5
2. SBP > 90 mm Hg 3. Euvolemia 4. Initial PaCO2 40 +/- 5 mmHg 5. Preoxygenate to maintain PaO2 >200 mmHg 6. Deliver 100% O2 during test via cannula in trachea 7. Stop ventilator; test positive if no respirations over 8-10 minutes, provided that PaCO2 > 60 mmHg, and 20 above baseline. 8. pH < 7.28. 9. Witnessed continuously by 2 licensed physicians experienced in determination of brain death. |
|
|
|
What is the alveolar gas equation?
|
Alveolar oxygen content comes from the the content of oxygen in the inspired air. Oxygen in the alveoli is displaced by CO2 diffusing from the blood. So, alveolar oxygen content is simply the difference in oxygen in inspired gas (air), and CO2 from the blood:
PAO2 = PIO2 - (PaCO2/R) The content of oxygen in the inspired gas depends on the FiO2, the atmospheric pressure, and the amount of water vapor in the gas: PAO2 = (FiO2 x (Patm - PH2O)) - (PaCO2/R) At sea level, the Patm is 760, and the PH20 is 47. The RQ at rest is usually about 0.8: PAO2 = ((FiO2 x (760-47) - (PaCO2/ 0.8) PAO2 = (FiO2 x 713) - (PaCO2/0.8) |
|
|
|
What is the equation for the dead space fraction?
|
Vd/Vt = (PaCO2 - PECO2)/PaCO2
|
|
|
|
What is the equation for arterial oxygen content?
|
CaO2 = (SaO2 x 1.34 x [Hgb]) + (.0031 x PaO2)
|
|
|
|
What is the equation for static compliance?
|
Cst = Vt/(Pplat-PEEP)
|
|
|
|
What is the equation for resistance to airflow?
|
Rrs = (Ppeak - Pplat)/FLOW
|
|
|
|
Name two features that suggest cyanide toxicity from nitroprusside.
|
* Anxiety, agitation, tachycardia, myocardial ischemia, met. acidosis, hyperventilation, seizures, paradoxical hypertension.
*Elevated mixed venous O2, arteriolization of retinal veins or venous blood on venipuncture. *Usually assoc. w/ infusion rates > 10 mcg/min. |
|
|
|
Name four treatments for cyanide toxicity
|
*100% O2.
*Amyl and sodium nitrite - induce formation of methemoglobin which binds cyanide. Can reduce O2 carrying capacity in burns with concomitant carboxyhemoglobin. *Sodium thiosulfate enhances conversion of cyanide to thiocyanate (can be coadministered with nitroprusside to eliminate chance of toxicity). *Hydroxycobalamin (B12 precursor) - increases urinary cyanide excretion. *EDTA - binds cyanide potently. SE's include arrhythmias, hypotension, nausea, vomiting, allergic rxn. *Remove contaminated clothing, gastric emptying for acute ingestions, followed by AC (not induced emesis). *Dialysis only effective to clear thiocyanate (formed with sodium thiosulfate). *HBO |
|
|
|
How quickly do you reduce BP from 180/110 to 120/80 with a hypertensive aortic dissection?
|
Use antihypertensives to reduce SBP and pulse wave (dP/dTmax). SBP should be reduced to levels that will halt the progression of the dissection. Goal 90-100mmHg with adequate organ perfusion (sensorium, U/O, lack of lactic acidosis)
|
|
|
|
Name five management strategies for RV infarct.
|
1. Cautious fluid bolus (eg. 250 mL) if pt. is dry, but otherwise avoid overresusc and R heart distension.
2. Dobutamine once pt. is not hypovolemic. 3. Inhaled NO. 4. IABP to combat incr. R sided wall stress and O2 demand, and improve R coronary perfusion. 5. Norepinephrine as a systemic vasoconstrictor and positive inotrope may raise R coronary perfusion pressure. 6. Coronary reperfusion via thrombolytics or cath. 7. Avoid drugs that decrease preload (morphine, nitrates). 8. Avoid high PEEP. 9. Target high SaO2 (>96%) to minimize hypoxic alveolar vasoconstriction (HAV). 10. Use the lowest tidal volume necessary to effect adequate elimination of CO2, but avoid hypercapnea (and attend pulmonary vasoconstriction). |
|
|
|
What is the probable diagnosis and treatment of a post-cardiac surgery patient with low cardiac output and high filling pressures?
|
1. Dx - cardiac tamponade until proven otherwise. Consider other R heart syndromes (PE, RV infarct, acute pulmonary hypertension).
2. Tx - supportive care, inotropes, vasopressors, echo, pericardiocentiesis ASAP. Resternotomy ASAP: Bedside or OR |
|
|
|
Name four specific causes of GI bleed in pancreatitis. Include at least two unrelated to gastritis or erosions.
|
1. Gastritis and associated gastric ulceration and bleed.
2. Rupture of a splenic arterial pseudoanuerysm. 2a. Rupture of a gasto-duodenal artery pseudoaneurysm. 3. Ruptured gastric varices due to splenic vein thrombosis and portal hypertension. 4. Erosions of local vessels due to pancreatic enzyme release and autodigestion. 5. Venous bleeding post necrosectomy. 5a. Venous bleeding into pseudocyst. 6. General oozing post surgical intervention. |
|
|
|
Name two non-infectious causes of shock and respiratory failure in a patient who is day 12 post BMT.
|
1. Acute transfusion reaction.
2. Pulmonary hemorrhage associated with thrombocytopenia. 3. Acute graft vs. host disease. 4. Cardiac failure assoc. w/ chemo. 5. Diffuse alveolar hemorrhage syndrome (DAH - BAL get lots of fluid, increasing blood on progressive samples, hemosiderin laden macrophages). |
|
|
|
In a multiparous labouring women who develops a sudden onset of hypoxemia and shock, what are 4 DDx? If she then develops coagulopathy and thrombocytopenia, what is the most likely Dx.
|
1. Amniotic fluid embolism.
2. Pulmonary embolism, given DVT increased risk during pregnancy. 3. Hemorrhagic shock associated with labour (eg. uterine rupture, retained placenta, uterine inversion, uterine atony). 4. DIC. 5. Abruptio placenta. 6. Placenta previa and associated massive hemorrhage - more common in multiparous women. 7. Septic shock. 8. Pregnancy induced cardiomyopathy. 9. Aortic dissection induced by labor. Part two of the question - DIC may be associated with amniotic fluid embolism, other causes of massive hemorrhage, or be idiopathic. |
|
|
|
What are the three most common causes for hypotension following intubation in status asthmaticus?
|
1. DHI and increased intrathoracic pressure, generally due to overenthusiastic bag-mask ventilation.
2. Sedation and associated decreased sympathetic tone. 3. Hypovolemia due to high insensible losses and decr. oral fluid intake. 4. Tension pneumothorax. |
|
|
|
What are the major determinants of air trapping in asthma?
|
1. Expiratory time (determined by minute ventilation and inspiratory flow rate.)
2. Tidal volume. 3. Degree of airway obstruction (determined by mucous plugging, inflammation, airway wall swelling, smooth muscle tone). |
|
|
|
Name the clinical features and two treatments of serotonin syndrome?
|
Clinical features - various combinations of confusion, agitation, coma, shivering, flushing, hyperthermia, diaphoresis, mydriasis, tachycardia, DIC, convulsions, muscle rigidity, myoclonus, hyperreflexia, involuntary movements, autonomic instability, nausea, diarrhea, orthostatic hypotension, rhabdomyolysis.
Broader categories - altered LOC, hypermetabolism, sympathetic tone, muscular rigidity, hyperreflexia, rhabdo. Tx - gastric emptying in acute ingestion, AC, cathartic, benzos. In severe cases, may consider serotonin antagonists (methysergide, cyproheptadine). Mechanical ventilation, cooling, neuromuscular blockade prn (esp if temp >41 deg) Different from NMS by quicker onset symptoms, hyper reflexic |
|
|
|
In a patient who has had a TEE with benzocaine spray as a topical anesthetic, who then becomes hypoxemic, what is the diagnosis and treatment?
|
Methemoglobinemia.
Dx; co-oximetry levels of methemoglobin, chocolate venous blood, gap between measured and finger sats. Tx; Removal of inciting drug or toxin (gastric emptying, AC, removal of clothes, washing of skin, etc.). Methylene blue 1-2 mg/kg acts over 30-60 minutes. Contraindicated in G6PD, renal failure. Additional therapies in severe conditions; PLEX, hyperbaric O2. |
|
|
|
List four predictors of outcome in out-of-hospital arrest that can be found on EHS records.
|
1. Initially documented rhythm (VF/VT 10-15 times better survival than PEA/asystole).
2. Presence or absence of return of spontaneous circulation after full resuscitation protocol. 3. Witnessed or unwitnessed. 4. Shocks delivered or not. 5. Response interval (> 8 minutes is assoc. with poor prognosis). 6. Hypothermia on arrival. "The authors found that only 0.5 percent of patients with an arrest survived if there was no return of spontaneous circulation, no shocks were administered, and the arrest was not witnessed by EMS personnel. When a “response interval greater than eight minutes” was retrospectively added to the prediction rule, the survival rate was 0.3 percent, and when “not witnessed by a bystander” was added, no such patients survived." |
|
|
|
Name four things that reduce CRBSI.
|
1. Proper hand hygiene before and after manipulation of catheter or insertion site.
2. Use of hat, mask, sterile gown and gloves, large sterile drape during insertion. 3. Aseptic technique during insertion and care. 4. 2% chlorhexidine for insertion-site antisepsis. 5. Use of transparent semipermeable dressing to cover insertion site. 6. Removal of non-essential catheters. 7. Do not replace infected catheters over a guidewire. (dont the new guidlines say you can?) 8. Subclavian insertion site when possible. |
|
|
|
What are recommended strategies to reduce VAP?
|
1. HOB at 30-45 degrees when on ventilator or receiving enteral tube feeds.
2. D/C mechanical ventilation and enteral feeds as soon as clinically feasible. 3. Hand hygiene before and after contact with pt., resp. devices, or objects contaminated with resp. secretions. 4. Periodic drainage of condensate from tubing (away from patient). 5. Sterile water in nebulizers and humidifiers, and to remove secretions from patient. 6. Sterilize equipment between patients (ambu-bag, portable respirators, oxygen or CO2 sensors, ventilators). 7. Subglottic suction tubes. 8. Oral decontamination with chlorhexidine. |
|
|
|
Ca++ channel blocker overdose.
|
1. Supportive care.
2. Atropine. 3. Pressors. 4. Calcium. 5. Glucagon. 6. Fluid. 7. Insulin and D50. 7.5 Phosphodiesterase inhibitor. 8. IABP. 9. Bypass/Ecmo. |
|
|
|
How would you place an NJ tube in a patient on an oscillator after an initial blind attempt failed?
|
Other options that can be done at the bedside, given that patient is on oscillator:
-cool tube to stiffen prior to placement. -use laryngoscope, glidescope to visualize tube entry into esophagus. -sit pt. up. -endoscopic placement. -fluoroscopic placement. -re-try with blind attempt with more experienced operator. |
|
|
|
What is the diagnosis in a patient with post-extubation laryngospasm or tube biting who develops pulmonary edema? How do you make the diagnosis? What is the mechanism? What is the protein content of alveolar fluid compared to plasma?
|
-Negative pressure pulmonary edema.
-Related to the generation of markedly negative intrathoracic pressure due to forced inspiration against a closed glottis, referred to as the Mueller (or reverse Valsalva) maneuver. This results in transudation of fluid from pulmonary capillaries to the interstitium following relief of the upper airway obstruction. May be exacerbated by increased pulmonary capillary permeability in post-surgical patient. -Fluid should be transudative, therefore pleural fluid protein/serum protein ratio should be < 0.5. Also the LDH ratio should be < 0.6, and the LDH content should be <2/3 the upper limit of normal for serum LDH. |
|
|
|
In a Crohn's patient with calculated energy requirements of 2400 kcal/d, how much protein, carbohydrate, and fat should you provide?
If the patient was severely malnourished, how many calories would you provide in the first day? Name two electrolytes you would check regularly in the first 48 hours. |
Think: "20,50,30, PRO, CHO, FAT".
i.e., split energy requirements into 20% protein, 50% carbohydrate, and 30% fat. TF, this patient needs: 20% of 2400 kcal protein = 480 kcal protein. 50% of 2400 kcal carbohydrate = 1200 kcal carbohydrate. 30% of 2400 kcal fat = 720 kcal fat. Next, you need to convert the kcal to grams based on energy densities: Then you have to remember "4.0, 3.4, 10" as the energy densities of PRO, CHO, Fat, in kcal/gram. Do the math, and you'll find that this patient needs 120 g protein, 352 g carbohydrate, and 72 grams of fat per day. Finally, you can convert to volumes of solution, if you remember standard solution concentrations: 10% AA = 10 g PRO/100 ml D50W = 50 g CHO/100ml 20% lipid = 20 g FAT/100 ml Again, doing the math, (after rounding off grams) you find the patient needs about: 1200 mL of 10% AA sol'n 700 mL of D50W 350 mL of 20% lipid solution. To minimize the risk of refeeding syndrome, start feeding slowly at about 20 kcal/kg/day, and increase slowly depending on absorption, electrolytes. Monitor K+, Mg++, PO4-- levels closely, as they can become severely low in refeeding syndrome and lead to arrhythmias. |
PRO/CHO/FAT
20/50/30 4/3.4/10 kcal/g 10/50/20 g/100mL |
|
|
What predicts myopathy in vented status asthmaticus?
|
Steroids + paralytics.
Prolonged neuromuscular blockade. Paralytics + aminoglycosides. ?B-agonists. |
|
|
|
Brain dead donor in shock, low cap refill, CVP 9. What drug should be instituted? Why? What 2 other drugs will improve myocardial function in this setting?
|
Goal CVP 6-10, so don't add any more fluid. Instead, add dopamine (<10 mcg/kg) as per Canadian guidelines a priori recommendation for inotropic support. Other drugs that might improve myocardial function are basically combined hormonal therapy, i.e.
• Thyroid hormone (tetraiodothyronine or T4), 20 µg IV bolus followed by 10 µg/h IV infusion • Vasopressin, 1 U IV bolus followed by 2.4 U/h IV infusion (max dose 0.04 U/min). • Methylprednisolone, 15 mg/kg IV every 24 h. Note CMAJ supliment March 14 2006. recommendation 2.4 "Fist line support for hemodynamic support)- vasopressin |
|
|
|
What are contraindications to TIPs?
|
Severe encephalopathy.
Sepsis. Right sided heart failure with elevated CVP. Pulmonary hypertension. Polycystic liver disease. Portal vein thrombosis. Unrelieved biliary obstruction. Budd Chiari. Lack of venous access. Hepatoma. Severe coagulopathy, thrombocytopenia. |
|
|
|
What are the complications of TIPS?
|
Bleeding.
New or worsened encephalopathy. Transcapsular puncture. Intraperitoneal bleed. Hepatic infarction. Hemobilia. Sepsis. Hemolysis. Stent migration, thrombosis or stenosis. |
|
|
|
What drug characteristics will influence clearance when patients are initiated on CVVHD?
|
How albumin bound they are (protein binding).
Volume of distribution. Molecular weight (500-5000 daltons will cross the membrane). Ionic charge. |
|
|
|
Features of propofol infusion syndrome.
|
Metabolic acidosis.
Rhabdomyolysis. Cardiac depression. Renal failure. 75 mcg/kg/hr for more than 72 hours - case series. Disadvantages: lipid content - calories, hypotension, cardiac supression, cost, venodilation, increased infection risk. |
|
|
|
Label EDV, ESV, and SV on PV curve, show how to calculate EF. Draw a PV curve with increased afterload with unchanged preload and contractility.
|
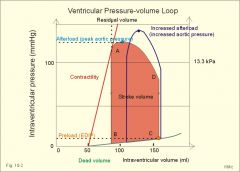
See picture.
|
|
|
|
Calculate PPV, NPV from test
|
Show 2x2 table.
A/A+B D/C+D |
|
|
|
Calculate odds ratio.
|
The odds ratio is a way of comparing whether the probability of a certain event is the same for two groups.
An odds ratio of 1 implies that the event is equally likely in both groups. An odds ratio greater than one implies that the event is more likely in the first group. An odds ratio less than one implies that the event is less likely in the first group. OR=(EE / EN) / (CE / CN) |
|
|
|
Calculate ARR, AHI (absolute harm increase), NNT, NNH.
|
ARR = Percent - percent.
RRR = Difference in percent divided by the larger percent. AHI = Percent - percent. NNT = 1/ARR NNH = 100/AHI percent. |
|
|
|
Other than plasma or vit K, how can you quickly reverse coumadin anticoagulation.
Name two products you could give. |
Octaplex. contains:
Factor II, VII, IX, X Protein C, S Heparin Recombinant factor VIIa |
|
|
|
What soluble clotting factors are in plasma other than vit K ones?
|
Vit K: 10, 9, 7, 2 (Prothrombin), protein C, protein S.
Non Vit K: Fibrinogen, tissue factor, 5, 8, 11, 12, 13, von Willebrand factor, prekallikrein, high molecular weight kininogen, fibronectin, ATIII. |
|
|
|
What are the features of botulism (woman and husband in Northern Ontario ate fish)? Name 4 differential diagnoses of someone presenting with these symptoms.
|
Botulism - nausea, vomiting, anticholinergic symptoms (dry mouth), bulbar symptoms, possible resp obstruction and distress, descending paralysis, symmetrical, flaccid, no sensory deficits. Toxin prevents release of ACh at the NM junction.
Treatment; AC if presenting early, equine antitoxin, MV prn. Aminoglycosides and clindamycin contraindicated because of their ability to increase blockade. Atropine is not indicated. The differential diagnosis for food-borne, wound, and adult enteric botulism includes: myasthenia gravis, Lambert-Eaton myasthenic syndrome (LEMS), tick paralysis, Guillain-Barré syndrome, poliomyelitis, stroke, and heavy metal intoxication. Less likely diagnoses include tetrodotoxin and shellfish poisoning and antimicrobial-associated paralysis. |
|
|
|
Prolonged delivery, petechiae, thrombocytopenia, coagulopathy, pulmonary infiltrates. Dx?
|
Dx – amniotic fluid embolism
DDx, HELLP,sepsis, aspiration pneumonia, cardiomyopathy |
|
|
|
Definitions of:
Bias Confounding Type I error Type II error |
Bias - external influences that may affect the accuracy of statistical measurements (not random).
Confounding - a confounding variable (also confounding factor, lurking variable, a confound, or confounder) is an extraneous variable in a statistical model that correlates (positively or negatively) with both the dependent variable and the independent variable. Type I (α): reject the null hypothesis when the null hypothesis is true Type II (β): failing to reject the null hypothesis when the null hypothesis is false |
|
|
|
Trauma patient; transfused 2 units of blood, hours later DIC. Dx, DDx? 2 lab tests to determine cause?
|
ABO incompatibility transfusion reaction, minor antibody, infected bag, fat embolism, brain injury (prone to coagulopathy), related to injuries (? massive trauma).
INR/PTT prolonged, fibrinogen down, peripheral smear (?MAHA associated with ABO incompatibility), direct antiglobulin test (Coombs test), plasma free Hgb, urine for Hgb, recheck bag for typing, platelets down, D-dimer high. |
|
|
|
Diagnostic criteria for hepatorenal syndrome?
|
*Chronic or acute hepatic disease with advanced hepatic failure and portal hypertension
*A plasma creatinine concentration above 1.5 mg/dL (133 µmol/L) that progresses over days to weeks. The rise in plasma creatinine with reductions in glomerular filtration rate may be minimized by the marked reduction in creatinine production. *The absence of any other apparent cause for the renal disease, including shock, ongoing bacterial infection, current or recent treatment with nephrotoxic drugs, and the absence of ultrasonographic evidence of obstruction or parenchymal renal disease. It is particularly important to exclude spontaneous bacterial peritonitis, which is complicated by acute renal failure that may be reversible in 30 to 40 percent of patients. *Urine red cell excretion of less than 50 cells per high power field (when no urinary catheter is in place) and protein excretion less than 500 mg/day. *Lack of improvement in renal function after volume expansion with intravenous albumin (1 g/kg of body weight per day up to 100 g/day) for at least two days and withdrawal of diuretics. |
|
|
|
What treatment options exist for hepatorenal syndrome other than transplant?
|
* Midodrine 7.5-12.5 mg tid + Octreotide 100-200 mcg SC tid (vasoconstrictor effect + inhibition of endogenous vasodilator release).
*Norepi + albumin (less evidence). *Early evidence shows possible efficacy of vasopressin analogs. *TIPS may provide short term benefit as a last resort. *Dialysis can be used as a bridge to transplant or when hepatic failure is expected to improve. |
|
|
|
In a patient with a traumatic brain injury and diffuse axonal injury:
What is the goal CPP? What are indications for ICP monitoring? What is the recommended duration of seizure prophylaxis? Is hyperventilation indicated? |
1. Goal CPP between 50-70 mmHg (60 mmHg.)
2. GCS < 8, depressed skull fracture, intracranial blood, unstable (posturing, etc.), older than 40 with normal CT scan, SBP < 90 3. For any instability, low GCS, contusion, depressed skull fracture, guidelines recommend prophylaxis for 7 days. 4. No hyperventilation, except acutely as a bridge to definitive treatment (eg. surgery). Otherwise CO2 should be kept in low normal range. |
|
|
|
In a 45 yo woman with a fall and CT showing intraperitoneal blood, splenic injury, what are two treatments?
|
1. Splenectomy.
2. Angiogram and embolization. 3. If stable then non-op mgt |
|
|
|
In a post-op cardiac surgery patient diagnosed with tamponade, what treatment do you initiate while awaiting OR.
|
1. Increase PEEP to try and decrease bleeding.
2. Keep full to optimize venous return. 3. Inotropes prn. 4. Type, crossmatch, urgent OR. 5. Thoracotomy tray to bedside. |
|
|
|
In a patient post CABG with a wide mediastinum on CXR, and a PA catheter shifted to the left, what is the probable diagnosis? What might you find on echo?
|
Dx - tamponade.
Echo finding - RV collapse in diastole, RA, LA collapse in diastole, pericardial effusion. |
|
|
|
In a patient with chronic COPD admitted and ventilated to a normal CO2 (to 45), who has a pH of 7.6, what is the cause of the alkalosis?
|
Overventilated pt. with a chronic hypercarbia, elevated bicarb.
|
|
|
|
On a ventilator tracing with prolonged expiratory flow, what is the cause, and what are the treatments (ventilator treatments x 4)?
|
Cause - dynamic hyperinflation.
Treatment - inverse ratio, decrease rate, permissive hypercapnia, PEEP matching if breathing spontaneously, ZEEP if paralyzed, bronchodilators, square wave form, low tidal volumes, increased sedation, paralysis. |
|
|
|
What can an ICU director do to ensure standards are maintained?
|
1. Evaluate: monitor results (ICU database, M+M rounds, pt. outcomes, bouncebacks, critical events, patient satisfaction).
2. Educate: M+M rounds, QI projects, empower employees, provide time, funding for CME. 3. Maintain good relations among staff, provide importance of communication. |
|
|
|
In a 24 year old patient with CF who does not want intubation:
What are appropriate psychosocial interventions? What do you do when he fails? What is the relevant bioethics principle? |
Discuss with patient, family (if patient wants), social work, evaluate competence, possible psychiatric consult. Pt. needs free will, needs to understand consequences of decisions, and be able to relate them to you, not delerious or otherwise altered. Explore reasons behind decision, pt. plans, thought process, etc.
When he fails, palliate as per his wishes. The relevant bioethics principle is autonomy. Other principles are justice, non-maleficence, beneficence. |
|
|
|
Define ultrafiltration/convection
|
Convection / ultrafiltration – solute is carried (in solution) across a semipermeable membrane in response to a transmembrane pressure gradient (a process known as solute drag). This mimics what actually happens in the normal human kidney. The rate of ultrafiltration depends upon the porosity of the membrane and the hydrostatic pressure of the blood, which depends upon blood flow. This is very effective in removal of fluid and middle-sized molecules, which are thought to cause uremia. Moreover, most of the cytokines involved in sepsis are “middle molecules”.
Ultrafiltration is commonly used to refer to the process of fluid removal via a pressure gradient, whereas convection refers to removal of solute via solute drag. (eg. SCUF and CVVHDF) |
|
|
|
Define dialysis/diffusion
|
Solute is moved across a semi-permeable membrane in response to a concentration (electrochemical) gradient. (eg. CVVHD).
|
|
|
|
For a patient with hepatic and renal failure on CVVHDF running with citrate anticoagulation, why might they become hypocalcemic? What is the management of the hypocalcemia?
|
Citrate accumulation in the patient may occur due to decreased metabolic clearance by the liver. The citrate binds calcium, thereby causing hypocalcemia. Management would include the following: decrease the concentration of citrate running in the arterial limb of the dialysis machine, increase the replacement calcium concentration, or switch to alternative anticoagulation (heparin), or no anticoagulation, such as in high flow, no anticoagulation, dialysis.
Accepted calcium post-filter 0.35-0.45. |
|
|
|
How do you differentiate aberrant SVT from VT at the bedside (other than on EKG)? 2 markers
|
Valsalva maneuver should slow SA nodal activity and AV nodal conduction via increased parasympathetic tone; may be done with other methods to increase intrathoracic pressure such as increased PEEP; will cause slowing of SVT.
Carotid sinus massage has a similar effect. Looking for cannon A waves on the JVP can also help identify VT, and will not be present in SVT. Variable S1 is also highly specific for VT (Garratt CJ et al, Circ 1994) |
|
|
|
Name two ways to terminate an aberrant SVT?
|
Carotid sinus massage, valsalva, ice.
Adenosine. Amiodarone, digoxin, diltiazem. Synchronized cardioversion. |
|
|
|
If the end of a pressure tubing is placed in water, and zeroed and levelled, what is the pressure reading at the zero level? What if the tubing is moved 10 cm lower in the column of water?
|
Pressure reading at zero level - 0 cm water. Pressure reading 10 cm deeper 0 cm water, because the pressure transducer is still at the surface.
|
|
|
|
Name four things that will shift the oxyhemoglobin dissociation curve to the right. How does this affect tissue oxygenation.
|
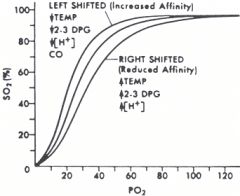
The curve shifts right with increases, and left with decreases in the following factors:
Temperature [H+] 2,3-DPG PCO2 Increasing CO concentration shifts the curve to the left (increased affinity, decreases tissue oxygenation). Increasing [Fetal hemoglobin] shifts curve to the left (increased binding affinity for O2, less dissociation). Shifts to the right decrease hemoglobin's affinity for oxygen, so increase tissue oxygenation. Opposite to the left. |
|
|
|
In a clinical trial where 20% of the controls survive, and 50% of the intervention subjects survive, what is the:
RRR? ARR? OR? |
RRR = (EER-CER)/CER = (0.5-0.8)/0.8 = -0.375
ARR = EER-CER = 0.5-0.8 = -0.3 OR = (EE/EN)/(CE/CN) = (50/50)/(80/20) =1/4 = .25 |
Set up 2 x 2 table.
Find experimental events (EE), experimental non-events (EN), control events (CE), control non-events (CN), experimental event rate (EER), control event rate (CER) to solve for ARR, RRR, OR. |
|
|
Define RRR in terms of control and experimental events. Show 2x2 table.
|
RRR = (EER-CER)/CER
|
|
|
|
Define ARR in terms of control and experimental events. Show 2x2 table.
|
ARR = EER-CER
|
|
|
|
Define OR in terms of control and experimental events. Show 2x2 table.
|
OR = (EE/EN)/(CE/CN)
|
|
|
|
Define RR in terms of control and experimental events. Show 2x2 table.
|
RR = EER/CER
|
|
|
|
Define EER in terms of control and experimental events. Show 2x2 table.
|
EER = EE/ES where EE = experimental events, and ES = experimental subjects.
|
|
|
|
Define CER in terms of control and experimental events. Show 2x2 table.
|
CER = CE/CS where CE = control events, and CS = control subjects.
|
|
|
|
Define NNT in terms of control and experimental events. Show 2x2 table.
|
NNT = 1/ARR = 1/(EER-CER)
|
|
|
|
In a patient with a traumatic spinal cord injury who has deltoid and biceps function, but no sensation below the nipples except for paresthesiae in the feet:
What is the grade? What is the management? |
In this case, ASIA Grade B (sensory, but not motor function below neurological level). Level is about C5. Other grades are ASIA A (complete) through E characterized by various degrees of motor function in muscles controlled by levels of the spinal cord caudal to the injury.
Management: ABCD. Spinal immobilization measures. Admit to intensive care. Monitor for resp./hemodynamic instability. Baseline FEV1. Avoid hypotension. Goal MAP 85-90 x 7 days. Urinary catheter to bladder distension (stimulus for autonomic dysreflexia). DVT prophylaxis. No steroids, based on Canadian guidelines (some do, but evidence poor). Surgical management for instability. Early physio, chest physio, assisted cough. Watch for autonomic dysreflexia, treat for significant hyper/hypotension. Note: talked to my spine surgery buddy and he says parasthesias dont count for sensation so this guys is an ASIA A. |
|
|
|
List 2 factors that would influence antibiotic choice in bacterial meningitis.
|
1. Patient age (listeria more likely at extremes of age, therefore higher need for ampicillin).
2. Community prevalence of cephalosporin resistance in S. pneumoniae - higher prevalence increases need for Vancomycin. 3. Pt. immune suppression - need better gram negative coverage (eg. ceftaz vs ceftriaxone), and definite Listeria coverage (ampicillin). 4. Recent head trauma, surgery, or neurosurgical device - replace ceftriaxone with ceftaz due to greater risk of Pseudomonas/Acinetobacter. 5. Nosocomial infection - again ceftaz to cover pseudomonads. 6. CSF gram stain and culture results - tailor antibiotics to organisms present. |
|
|
|
In suspected bacterial meningitis, should steroids be used, or not. If so, when and why?
|
Early intravenous administration of glucocorticoids (usually dexamethasone) has been evaluated as adjuvant therapy in an attempt to diminish the rate of hearing loss, other neurologic complications, and mortality. Proposed mechanism is better crossing of blood brain barrier by antibiotics. The main indication for dexamethasone therapy in adults is known or suspected pneumococcal meningitis, particularly if the Glasgow coma scale score is 8 to 11. The usual regimen is 0.15 mg/kg every six hours for four days, beginning shortly before or at the same time as the first dose of antibiotics.
Dexamethasone should be discontinued if the Gram stain and/or cultures reveal another pathogen or if bacterial meningitis is subsequently thought not to be present. Dexamethasone should NOT be given to adults who have already received antimicrobial therapy, because it is unlikely to improve patient outcomes. |
|
|
|
What is the formula for the oxygenation index?
|
Oxygenation index = (Mean Airway Pressure x FiO2)/PaO2.
Normal ~ 0.5. Bad is around 5-8. |
|
|
|
Derive the formula for the classic shunt equation.
|
Total oxygen delivery (QO2) is the product of the cardiac output (Qt) and the arterial oxygen content (CaO2), like this:
QO2 = Qt x CaO2. If you assume there are only two kinds of pulmonary perfusion (either 100% shunt or 0% shunt), then the total cardiac output is split into either blood flowing through oxygenated capillaries (Qc), or shunted blood (Qs), like this: Qt = Qc + Qs. This can be rearranged to solve for Qc, like this: Qc = Qt - Qs. We will need to substitute this into the shunt equation in a bit. Similarly, the total oxygen delivery can be split into that delivered to oxygenated capillaries and shunted capillaries, like this: Qt x CaO2 = Qc x CcO2 + Qs x CvO2. Now, substitute for Qc from above: Qt x CaO2 = (Qt-Qs) x CcO2 + Qs x CvO2. Multiply out the brackets: Qt x CaO2 = Qt x CcO2 - Qs x CcO2 + Qs x CvO2. Rearrange to get the Qs and Qt expressions on the same side: Qt x CaO2 - Qt x CcO2 = -Qs x CcO2 + Qs x CvO2. Multiply everything by -1. Qt x CcO2 - Qt x CaO2 = Qs x CcO2 - Qs x CvO2 Factor out the Q terms: Qt x (CcO2-CaO2) = Qs x (CcO2-CvO2) Rearrange to find Qs/Qt for shunt fraction: Qs x (CcO2-CvO2)/Qt = CcO2 - CaO2 Qs/Qt = (CcO2-CaO2)/(CcO2-CvO2) |
|
|
|
Once you've derived the shunt equation, how do you find the CaO2 and CvO2 to supply into the equation?
|
The generic formula for any oxygen content is the sum of the oxygen bound to hemoglobin, and the oxygen dissolved in blood, like this:
CxO2 = 1.34 x SxO2 x [Hgb] + .0031 x PxO2. So, the CaO2 is: CaO2 = 1.34 x SaO2 x [Hgb] + .0031 x PaO2 And, the CvO2 is: CvO2 = 1.34 x SvO2 x [Hgb] + .0031 x PvO2 |
|
|
|
Once you've derived the shunt equation, how do you find the CcO2 to supply into the equation?
|
The generic formula for any oxygen content is the sum of the oxygen bound to hemoglobin, and the oxygen dissolved in blood, like this:
CxO2 = 1.34 x SxO2 x [Hgb] + .0031 x PxO2. So, for the CcO2 CcO2 = 1.34 x ScO2 x [Hgb] + .0031 x PcO2. Given that oxygenated capillaries are by definition in direct contact with alveoli, we assume that their SaO2 is 100%, or 1 for the equation, so that term can be left out, like this: CcO2 = 1.34 x [Hgb] + .0031 x PcO2. We can't directly measure PcO2, but again, given direct contact with the alveoli, and assuming no diffusion impairment, we can substitue the PAO2, so: CcO2 = 1.34 x [Hgb] + .0031 x PAO2. The PAO2 then comes from the alveolar gas equation: PAO2 = FiO2 x (Patm-PH20) - PaCO2/RQ. |
|
|
|
Draw a pressure volume curve for the lung. Mark the lower and upper inflection points.
|
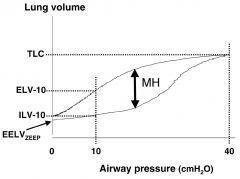
|
|
|
|
On a pressure volume curve for the lung, how do you determine the compliance.
|
Compliance is the change in volume for the change in pressure (C = delta V/ delta P). On a ventilator, the static compliance is the tidal volume divided by the difference between the PEEP and plateau pressures. On the pressure volume curve, a more compliant lung has a greater rise in volume for a given pressure change, so the slope of the curve is steeper. A less compliant lung has a shallower slope.
|
|
|
|
On a pressure volume curve for the lung, how do you determine the compliance.
|
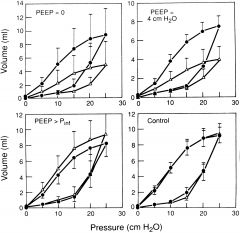
Compliance is the change in volume for the change in pressure (C = delta V/ delta P). On a ventilator, the static compliance is the tidal volume divided by the difference between the PEEP and plateau pressures. On the pressure volume curve, a more compliant lung has a greater rise in volume for a given pressure change, so the slope of the curve is steeper. A less compliant lung has a shallower slope.
|
|
|
|
Name strategies, factors known to reduce the rate of line sepsis.
|
-Sterile technique when placing catheters, including removing jewellery, washing hands, wearing a surgical cap, mask, and gown, cleaning the insertion site with 2% chlorhexidine, allowing the prep. solution to dry, using a full body drape.
-Sterile technique when handling lines, including covering with a clear dressing, doing daily line care, using alcohol swabs when accessing lines. -Removing lines, and replacing them either over a guidewire or resiting if line sepsis suspected. -Daily review to ensure ongoing necessity of lines, and prompt removal when line no longer necessary. -Switching to tunnelled or PICC lines if longterm access is necessary. -Consider use of antibiotic impregnated lines for lines lasting > 5 days. |
|
|
|
What is the mechanism of improved oxygenation with prone positioning?
|
Prone positioning can improve oxygenation owing to several mechanisms that improve V'/Q', in general, and consequently cause a reduction in physiological shunt. These include increased lung volume, redistribution of perfusion, recruitment of dorsal lung regions and a more homogeneous distribution of ventilation. (Gattinoni 2002)
-Increased lung volume. -Redistribution of perfusion. -Recruitment of dorsal lung regions. -More homogeneous distribution of ventilation (decreased pleural pressure gradient). -Enhanced clearance of secretions. |
|
|
|
In a patient with an accidental overdose of inhaled NO, what is your management?
|
Discontinuation of the agent.
Blood pressure support with pressors as necessary. High FiO2 if cyanotic. Methylene blue 1-2 mg/kg if methemoglobin levels are > 12%. May repeat in 60 minutes if necessary. Contraindicated in G6PD deficiency, renal failure. In severe methemoglobinemia, may consider exchange transfusion or hyperbaric O2. |
|
|
|
Name 2 mechanisms for tissue hypoxia caused by carbon monoxide.
|
1. Decreased saturated hemoglobin due to high affinity of hemoglobin for carbon monoxide (240 x greater than O2).
2.Shifts oxyhemoglobin dissociation curve to the left, interfering with off-loading of O2 in the periphery. 3. Blocks myoglobin facilitated diffusion of oxygen and myoglobin mediated oxidative phosphorylation, which results in impaired cardiac contractility. These effects are mediated by the binding of myoglobin to form carboxymyoglobin. 4. Inhibits enzymes of mitochondrial electron transport chain. 5. Inhibits intracellular enzymes (cyt P-450, NADPH reductase). Particularly problematic for tissues with high O2 consumption (heart, brain). |
|
|
|
Name 2 indications for hyperbaric oxygen treatment for carbon monoxide intoxication.
|
1. Severe CO poisoning (high levels, greater than 30% COHb).
2. Loss of consciousness. 3. Cerebellar dysfunction associated with CO poisoning. 4. No other life threatening injuries (burns, trauma), that would make time in hyperbaric chamber unsafe. 5. not responding to normobaric O2 |
|
|
|
What are the clinical characteristics of ICU delirium?
|
Delirium is defined as:
1. Acute onset of mental status changes or a fluctuating course. AND 2. Inattention. AND 3. Disorganized thinking. OR 4. Altered level of consciousness. |
|
|
|
Name a clinical scoring system for ICU delirium.
|
ICDSC (Intensive Care Delirium Screening Checklist).
DSM-IV CAM-ICU - i.e. the confusion assessment method for ICU. It assesses four key features: 1. Acute onset or fluctuating course. 2. Inattention. 3. Disorganized thinking. 4. Altered level of consciousness. |
|
|
|
Name 5 risk factors for the development of delirium in the ICU.
|
Patient factors:
-Elderly. -Pre-existing cognitive impairment. -Smoking, alcohol use. -Hearing or vision impairment. Disease factors: -Sepsis. -ARDS. -MODS. -Nosocomial infection. -Metabolic disturbance. Iatrogenic factors: -Use of benzodiazepines. -Dose dependant use of opiates. -Sleep deprivation. -Malnutrition. -TPN. -Immobilization. |
|
|
|
Name 5 clinical clues to suggest the possibility of an acute right heart syndrome (RV failure).
|
1. Hx of pulmonary hypertension.
2. Elevated neck veins. 3. Peripheral edema out of keeping with the degree of pulmonary edema. 4. Pulsatile liver. 5. Loud P2. 6. Increased P2 splitting. 7. Right sided S3. 8. Echocardiographic. 9. Electrocardiographic. 10. Radiographic. |
|
|
|
Discuss PA catheter findings in cardiac tamponade.
|
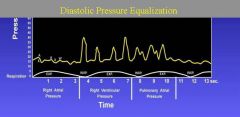
1. Equalization of diastolic pressures on the left and right sides of the heart.
2.Elevated right atrial pressure. 3. Kussmaul sign (ie, increase in right atrial pressure with inspiration). 4. Blunted y descent (in contrast to constrictive pericarditis and restrictive cardiomyopathy, which have a prominent y descent). |
|
|
|
What equalizes in pericardial tamponade?
|
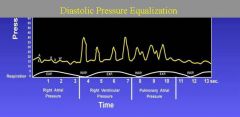
In cardiac tamponade, systemic arterial pressure (Pa) reflects pulsus paradoxus. Right atrial pressure (RAP) is elevated. Pulmonary artery (PA) diastolic pressure equals mean right atrial (RA), right ventricular (RV) diastolic, and wedge pressures.
|
|
|
|
In a patient with a history of pulmonary hypertension who is acutely ill, why might they become hypotensive? How can you address the elevated right sided pressures.
|
Patients with pre-existing PAH are at risk for acute increases in pulmonary pressures with acute illness, causing acute right heart syndromes.
This can cause hypotension via: 1. reduced RV output due to acute RV dilatation, moving it to a suboptimal portion of the starling curve, leading to decreased left ventricular filling pressures and cardiogenic shock. 2. ventricular interdependence whereby a dilated RV can cause septal shift, decreasing LV diastolic filling, and thereby decreasing cardiac output. 3. high right ventricular wall tension coupled with decreased cardiac output can decrease right sided coronary perfusion, causing right ventricular ischemia, possibly infarction, and further systolic dysfunction of the RV. Management of acute right heart syndromes includes: 1. Optimizing filling pressures with small boluses of 250 cc in a patient who is underfilled, but avoiding fluid boluses in patients who are already volume replete (high JVP, peripheral edema, etc.). 2. Dobutamine to increase cardiac output. 3. Norepinephrine to improve R coronary perfusion. 4. Avoidance of preload reducing medications (nitrates, morphine). 5. RV afterload reduction with inhaled nitric oxide, possibly inhaled flolan (direct pulmonary vasodilation). 6. Avoid high PEEP. 7. Target high SaO2 (>96%) to minimize hypoxic alveolar vasoconstriction (HAV). 8. Use the lowest tidal volume necessary to effect adequate elimination of CO2, but avoid hypercapnea (and attendant pulmonary vasoconstriction). |
|
|
|
How do you differentiate subtypes of aortic dissection?
|
Timing:
Acute: <2 weeks. Chronic: > 2 weeks. Mortality of acute is 1% per hour. Location: Type A: ascending aorta involved, independent of the site of the intimal tea. (60%) Type B: Descending aorta, distal to the left subclavian artery. (40%) |
|
|
|
In a hypertensive patient with an aortic dissection, what is your medical management of the hypertension?
|
Goal; reduction of SBP and the pulse wave (dP/dTmax).
1. IV Labetolol - reduces dP/dTmax via B-blockade and vasodilation. 2. Esmolol. 3. Nitroprusside and propranolol (if labetolol or esmolol not available). Must be given together to avoid sympathetic reflex increasing dP/dTmax with nitroprusside alone. Goal SBP 90-100 mmHg, as long as there is adequate cardiac output. Definitive management of the dissection is surgical for type A, possible endovascular stenting for type B. |
|
|
|
When flying at altitude, what are some possible detrimental occurrences?
|
1. Lower barometric pressure at altitude lowers the alveolar oxygen tension (think alveolar gas equation), and may cause or worsen hypoxemia (Dalton's law states that the partial pressure of any gas in a solution is determined by the fraction of that gas in the solution times the total pressure exerted by all the gases).
2. Lower barometric pressure causes expansion of trapped gas, so can worsen tension pneumothorax, cause bowel perforation in bowel obstruction, cause tracheal inujury due to endotracheal tube cuff expansion (Boyle's law states that the volume of a gas is inversely related to its pressure when temperature is held constant). |
|
|
|
List advantages and disadvantages of rapid sequence intubation.
|
Advantages:
1. Theoretically reduces risk of aspiration in patient with full stomach, by minimizing BVM ventilation. 2. Rapidly secures the airway. Disadvantages: 1. Requires paralytic, so removes patient ability to breathe spontaneously if airway cannot be established. 2. May be associated with post-intubation hypotension, as medications are not titrated to effect, but are bolus dosed. 3. May be associated with increased ICP if sedative dose is not sufficient in paralyzed patient. |
|
|
|
List five actions to be taken prior to passing an ETT.
|
Patient assessment:
1. Assess airway for possible difficult intubation. 2. Ensure adequate IV access for medication delivery both pre and post intubation. 3. Check BP and anticipate post-intubation hypotension - fluid boluses, pressors as necessary. Equipment 1. Check for all appropriate equipment - bag valve mask, suction, laryngoscope, ETT with stylet formed appropriately, end-tidal CO2 detector, back-up device in case of failure (bougie, LMA, etc.). Procedure 1. Pre-oxygenate. 2. Position patient appropriately. 3. Pre-medicate with induction agents (sedative hypnotic, analgesic, possible NMBA). 4. Visualize the cords using laryngoscope or other chosen method. |
|
|
|
Discuss considerations when intubating a patient with increased ICP.
|
1. Need to attempt not to increase ICP further with intubation; need appropriate sedation, consider avoidance of succinylcholine which may further increase ICP, consider addition of lidocaine to pre-medication, which may blunt ICP response to intubation.
2. Need to avoid hypotension post intubation with CHI, central neurologic injury, as a single episode of hypotension significantly worsens potential outcome. Watch for hypotension, consider art line prior to intubation, be prepared to institute vasopressors if necessary, give volume if necessary, etc. 3. Post-intubation, consider minimization of PEEP to minimize intrathoracic and secondary intracranial pressure. 4. Minimize any hypoxic episodes as worsens outcomes |
|
|
|
List 5 factors that might predict a patient will be difficult to intubate.
|
1. Hx of previous difficult intubations documented in chart.
2. Obesity. 3. Pregnancy. 4. Short neck. 5. Large tongue. 6. Inadequate mouth opening or TMJ dysfunction. 7. Small thyromental distance (small or recessed mandible). 8. Limited neck flexion. 9. Cervical instability. 10. Loose teeth. 11. Copious secretions or blood. 12. Mallampati 3 or 4 (i.e. soft palate or hard palate only visible; uvula not seen). 13. Facial trauma. |
|
|
|
List predictors of difficulty with BVM ventilation.
|
1. Facial hair.
2. Small or large face; difficult to fit mask properly. 3. History of difficulty with BVM ventilation. 4. Small mandible. 5. Copious secretions or blood. 6. Facial trauma. 7. no teeth or dentures |
|
|
|
What steps would you take to manage a patient with massive hemoptysis from the left lung awaiting surgical intervention?
|
1. Trendelenburg position may assist in clearing of blood from the airway.
2. Left lateral decubitus position to protect the opposite lung. 3. Small doses of codeine or morphine may be used to blunt cough response; need to exercise caution to preserve sensorium. 4. If necessary, secure airway - consider double lumen tube and/or selective right mainstem intubation. 5. Check coags - correct any coagulopathy. 6. Consider increased PEEP to try and tamponade bleeding. |
|
|
|
List methods to prevent VAP.
|
Positioning:
1. Elevate HOB to 45 degrees, or as high as possible. 2. Consider the use of rotating beds. Physical strategies 1. Use subglottic suction port ETT (Evac-tube) in patients expected to be intubated > 72 hrs. 2. Orotracheal intubation, as opposed to other routes. 3. New ventilator circuits for every patients, and change if becomes soiled or damaged. 4. Change airway heat and moisture exchangers between every patient, and every 5-7 days, or more frequently if clinically indicated. Pharmacologic strategies: 1. Consider the routine use of oral chlorhexidine. 2. Consider the routine use of oral povidone-iodine in head injured patients. |
|
|
|
What factors contribute to the development of MDR VAP?
|
1. Extended length of hospitalization.
2. Interhospital or nursing home transfer. 3. Invasive devices (i.e. central lines, mechanical ventilation). 4. Prior use of broad-spectrum antibiotics. 5. Previous recent hospitalization in institution with high endemic rates of MDR organisms. 6. Host immune supression. 7. High endemic rates of MDR organisms in ICU. 8. Advanced age. 9. Severity of illness. |
|
|
|
Name four mechanisms of antibiotic resistance in common ICU pathogens. Give examples.
|
1. Drug inactivation or modifications - eg. enzymatic inactivation of penicillin through the production of B-lactamases that destroy the beta lactam ring, seen for example in S. aureus resistance to penicillin.
2. Alteration of drug binding site - eg. alteration of PBP, the penicillin binding site in MRSA. 3. Alteration of metabolic pathway: e.g. some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides. Instead, like mammalian cells, they turn to utilizing preformed folic acid. 4. Reduced drug accumulation: by decreasing drug permeability and/or increasing active efflux (pumping out) of the drugs across the cell surface. 5. Prevents binding of abx to ribosomes so transcription continues. 6. There are three known mechanisms of fluoroquinolone resistance. Some types of efflux pumps can act to decrease intracellular quinolone concentration. In gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or Topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness. |
|
|
|
List causes and treatments of SBT failure.
|
Increased respiratory load:
1. Increased resistance - use bronchodilators, steroids, remove excessive airway secretions, treat upper airway obstruction. 2. Increased elastance (i.e. decreased compliance) : Treat pneumonia, pulmonary edema, DHI, Pleural effusions, pneumothoraces, ileus. 3. Increased minute volume: treat intrinsic PEEP, give bronchodilators, antipyretics, treat sepsis, PE, shock, avoid overfeeding. Decreased respiratory strength: -Replace electrolytes (K, Mg, PO4). -Treat sepsis. -Provide nutritional support. -Rule out neurologic disease, occult seizures, hypothyroidism, oversedation, critical illness myopathy/polyneuropathy. |
|
|
|
List methods to decrease ventilator dependancy time.
|
1. Assess patients daily for readiness of trial of extubation, using a systematic protocol.
2. Use PSV or T-piece trials as your preferential weaning method (as opposed to SIMV). 3. Daily SBT for all patients, either on T-piece or minimal PSV. |
|
|
|
When traditional ventilatory methods to maximize oxygenation (i.e. FiO2, PEEP) are at their maximum, what other methods are available to improve oxygenation? If possible, cite evidence associated with method.
|
1. Optimize oxygen delivery:
A. Patient methods: -Diurese if renal function and blood pressure tolerate. -Position patient with good lung down. -Prone position (Gattinoni, 1986). -Optimize cardiac output and MVO2 with fluid, transfusions if anemic, inotropes if cardiac dysfunction. -Drain pleural effusions. -CXR - R/O easily reversible causes - PTX, mucus plugging. -Consider other causes: PE, intracardiac shunt - get echo, bubble study, CT chest, etc. B. Ventilator methods: -Prolong I time: consider inverse ratio ventilation. -Slow respiratory rate, allow permissive hypercapnea. - Recruitment maneuver. -PCV. -HFOV. -Nitric oxide. -Inhaled prostaglandins (eg. Flolan) -Trial higher PEEP (LOVS and EXPRES)guided by esophageal balloon pressures.(Talmor 2008) -ECMO 2. Minimize oxygen consumption: -Sedate. -Paralyze prn. |
|
|
|
In a ventilated patient, what could cause a sudden increase in peak and plateau pressures?
|
-Coughing, straining against ventilator.
-Bronchospasm. -Auto-peep. -Mucous plugging, secretions. -Obstruction or kinking of the endotracheal tube,ventilatory tubing. -Tension pneumothorax. -Flash pulmonary edema. -Abdominal compartment syndrome. |
|
|
|
List possible causes of a rising PCO2 on an ABG, with no change in minute ventilation or airway pressures.
|
1. Increased Vd/Vt
-Increased ventilator tubing length. -Airway disease other than constrictive disease (eg. fungal infection of the airways). PE 2. Increased CO2 production -Overfeeding, esp. with CHO. -Sepsis. -Hyperthyroidism. -Fever -Potentially anxiety in paralyzed patient (will increase HR, metabolic rate, without changing Vt). |
|
|
|
Calculate airways resistance from ventilatory numbers.
|
Resistance to airflow (Rrs) =
deltaPressure/FLOW = Ppeak-Pplat/FLOW |
|
|
|
In a pregnant patient given tocolytics to slow labor, what are potential causes of acute pulmonary edema? What is the management?
|
-4.4 % of women given B-agonists (terbutaline, ritodrine, salbutamol), usually during or within 24 hours of administration. Higher risk with multiple gestation, infection, or corticosteroid Tx. Most common cause of pulmonary edema in pregnancy.
-pathogenesis unkown; unique to pregnant women. Thought to be due to pulmonary capillary leak in conjunction with volume overloading, reduced colloid oncotic pressure, impaired sodium and water excretion. -Management: discontinuationof the agent, oxygen and diuresis. Usually rapid response. -DDx: acute thromboemolic disease, aspiration, amniotic fluid embolism, cardiomyopathy of pregnancy. Hx and Px should differentiate. |
|
|
|
Name 5 clinical criteria for the declaration of brain death.
|
1. Complete lack of neurologic response, including movement, response to pain, brain stem reflexes.
2. No concurrent metabolic abnormalities that may account for decreased LOC (eg. endocrine, electrolyte, acid base disturbances). 3. No recent drug administration that may account for decreased LOC (eg. intoxicants, poisoning, paralytics, anesthetics, sedatives). 4. No spontaneous respiratory efforts made with apnea testing (CO2 rises to 60 from baseline 35-45, or 20 mmHg from baseline). 5. Cause of coma known and is adequate to explain irreversible cessation of brain function. 6. Core temperature >34 C. 7. Brain death exam to be confirmed by two physicians experienced in examination and declaration of brain death. |
|
|
|
List confirmatory tests for brain death.
|
1. Transcranial doppler ultrasonography.
2. Four-vessel cerebral angiography. 3. Radionuclide cerebral scanning. 4. MRI angiography (limited experience). 5. CT angiography (limited experience). |
|
|
|
In a brain dead patient who is awaiting organ donation, how would you manage central DI and hypotension?
|
Central DI:
-First line agent: vasopressin up to 0.04 U/min; will also help treat hypotension. -May add DDAVP if necessary. Upper limit of dose to be titrated to clinical picture. Normal dose 1-4 mcg IV, repeat every 6 hrs prn to maintain U/O < 4 ml/kg/hr. -Replace urine output 1:1 with hypotonic IV solution to maintain serum sodium 130-150. Hypotension: -Ensure volume resuscitation. -Vasopressin up to 0.04 U/min as first line agent. May also assist in treatment of DI. -Second line agents include norepi, epi, phenylephrine, dose titrated to MAP > 60. |
|
|
|
In a patient with DKA, why should you not simply normalize the blood sugar? (4 reasons).
|
1. Too rapid correction of osmolality can lead to cerebral edema.
2. Withdrawal of insulin will remove its anti-lipolytic effect, and promote the recurrence or persistence of ketogenesis. 3. Withdrawal of insulin may lead to rebound hyperglycemia, particularly if SC insulin has not yet been initiated. 4.Noncardiogenic pulmonary edema can result from the rapid reduction in colloid osmotic pressure. 5. Not sure if this is the gist of the question, but could also discuss the need for significant fluid replacement, careful monitoring of K+, PO4--, pH, renal function, etc. in the treatment of DKA. |
|
|
|
List four non-endoscopic predictors of mortality in upper GI bleeds.
Name 2 treatments to decrease rebleeding risk. |
Risk factors
-Age > 60 years. -Onset of bleeding during hospitalization. -Severe comorbidities. -Severe anemia requiring > 5U PRBCs. -Hypotension, clinical shock. -Red blood emesis or NG aspirate. -Emergency surgery. -History of cirrhosis. Treatments -IV bolus, then infusion of proton pump inhibitor (Pantoprazole) -correct coagulopathy; stop any anticoagulants, replace coagulation factors. -endoscopic treatment (banding, clipping, sclerotherapy). -surgical treatment. -consider TIPs if recurrent variceal bleeding. |
|
|
|
Describe the mechanisms of action of depolarizing and non-depolarizing neuromuscular blocking agents.
|
NMBAs bind to the postsynaptic motor end-plate acetylcholine receptor, thereby blocking the action of acetylcholine released from the presynaptic motor nerve.
Depolarizing agents (succinylcholine) activate the receptor, leading to depolarization of the nerve. Non-depolarizing agents competitively inhibit the receptor without activating it. |
|
|
|
What medication(s) can be used to reverse the action of NMBAs? What is the most concerning side effect of reversal, and what can be done to avoid it?
|
The effect of neuromuscular blocking agents can be reversed by the administration of a cholinesterase inhibitor, such as neostigmine (inhibits the breakdown of acetylcholine in the neuromuscular junction, thereby enhancing the effects of Ach and overwhelming the neuromuscular blockade) 0.035-0.07 mg/kg.
Acetylcholine-related side effects such as salivation, bradycardia, and cardiac standstill (the most concerning) are prevented by coadministration of atropine (15 mcg/kg) or glycopyrrolate (7 mcg/kg). |
|
|
|
Describe the findings of the vestibulo-ocular reflex in coma with an intact brainstem, vs. in a normal and awake patient.
|
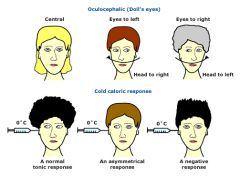
In a normal, awake patient:
Vestibulo-ocular reflexes are intact. Moving the head rapidly side to side results in the eyes staying midline, or fixed on a point of reference, as opposed to moving with the head turning. The same occurs for upwards/downwards movement of the head. Further testing using the oculo-caloric response results in nystagmus with cold water being injected into the ear, with the fast phase of nystagmus away from the ear being injected. In a comatose patient with an intact brainstem, movement of the head side to side or up and down will result in no change from the baseline exam; i.e. the eyes will remain midline, and will not deviate with head movement. The oculo-caloric response will be changed, however. The instillation of cold water into the ear will result in tonic deviation of the eyes to the side of instillation, with no rapid nystagmus away. In a comatose patient with no brainstem function, movement of the head will not have compensatory movement of the eyes; i.e. the eyes will move with the head, appearing "painted on". There will also be no deviation of the eyes with instillation of cold water into the ear canal. |
|
|
|
List lab and urine findings to differentiate pre-renal renal failure from ATN.
|
1. FeNa:
-pre-renal failure; <1%. -ATN >3%. 2. Urine Na+ -pre-renal failure - usually low. -ATN - normal or high. (depends on independent analysis of TBW). 3. Urine specific gravity: -pre-renal; high. -ATN; normal 4. U/A -Pre-renal; unremarkable, maybe occas. hyaline casts. -ATN; muddy brown granular casts, tubular cell casts, free renal tubular cells. 5. Response to volume: -Pre-renal - return to normal function in 24-72 hrs. -ATN - persistent renal dysfunction. 6.BUN/plasma Cr ratio: -Pre-renal - high; . 20:1 (active resorption of urea). -ATN - normal (10-15:1). 7. Serum Cr rise: -Pre-renal; slower, with fluctuations due to variability in renal perfusion. -ATN - faster, progressive. 8. Urine osmolality: -Pre-renal: high. -ATN:low. 9. Urine volume: -Pre-renal: low -ATN: may be oliguric or non-oliguric. |
|
|
|
List causes of low FeNa other than pre-renal failure.
|
-Nonoliguric ATN.
-Acute GN. -Acute partial urinary tract obstruction, or early complete obstruction. -AIN. -Contrast nephropathy. -Nontraumatic rhabdomyolysis. -Uric acid nephropathy. |
|
|
|
List methods to optimize clearance for a patient on dialysis.
|
-increase dialysate flow rate; consider use of IHD machine to maximize flows.
-change replacement fluids to post-filter if using CVVH, prefilter for CVVHDF. -minimize interruptions to dialysis. -increase ultrafiltration transmembrane pressure gradient. |
|
|
|
List common toxins and their antidotes.
|
-Acetaminophen: acetylcysteine
-Anticholinergics: physostigmine -Anticholinesterases: atropine -Benzodiazepines: flumazenil -B-blockers: glucagon -Black widow spider bites: equine antivenin -botulinum toxin - equine antitoxin -Ca+ channel blockers: CaCl, glucagons -Carbon monoxide: 100% FiO2, HBO -cocaine - benzodiazepines, alpha-adrenergic antagonist -Cyanide: amyl nitrite, sodium nitrite, sodium thiosulfate -Digoxin: digoxin specific antibody fragments (Fab, digibind) -Ethylene glycol: fomepizole, ethanol -Heavy metals (arsenic, copper, gold, lead, mercury): Dimercaprol, EDTA, penicillamine -Hypoglycemic agents: Glucagon, dextrose -Iron: Deferoxamine -Isoniazid: pyridoxine -isopropyl alcohol - fomepizole, ethanol not indicated, as metabolites nontoxic -Methanol: fomepizole, ethanol, folinic acid -Methemoglobinemia: Oxygen, methylene blue, exchange transfusion, HBO -Opioids: narcotics - naloxone 0.4 to 0.8 mg IV, IM, SC, up to 6-10 mg (narcan) -organophospates - atropine, 2PAM aka pralidoxime (SLUDGE - salivation, lacrimation, urination, diarrhea, GI cramping, emesis) -PCP - pinpoint pupils with agitation - haldol, benzos. -theophylline - charcoal hemoperfusion. -Rattlesnake bites: equine antivenin -TCAs - Na bicarb, lidocaine for ventricular arrythmias -Universal antidote: oxygen,dextrose, thiamine, narcan. |
|
|
|
In a patient with symptoms consistent with toxic alcohol poisoning, how do you calculate the osmolar gap?
|
Osmolar gap = measured osmolatlity - calculated osmolaltiy.
Measured serum osmolality comes from the labs. Calculated osmolality = (2 x Na) + BUN + Glucose. Normal osmolar gap is < 10. Higher than that suggests there are significant molecules with osmolar activity not accounted for by Na, Cl, Bicarb, Urea and Glucose. Examples of such osmoles would be ETOH (some include this in the calculated osmolality, although it does not actually account for 1:1 increase). Other osmoles include toxic alcohols (methanol, ethylene glycol, isopropyl alcohol), mannitol, sorbitol, IVIG in maltose solution. |
|
|
|
Discuss the presentation and management of the toxic alcohol poisonings.
|
Presentation:
Methanol - distinguishing features: optic papillitis, pancreatitis. Tx with AC +/- GL if early, ethanol, fomepizole 15 mg/kg bolus then 10 mg/kg q 12 hrs, HD if high serum levels refractory acidosis, end-organ damage. Folinic acid. Ethylene glycol - distinguishing features: crystalluria (oxylate), renal failure, wood's light urinary flourescence, myocardial dysfunction. Tx is as for methanol, with addition of thiamine, pyridoxine, calcium administration. Isopropyl alcohol - distinguishing features: hemorrhagic gastritis, ketonemia, ketonuria, no acidosis or hyperglycemia. Tx is as above, but no need for EtOH or fomepizole (nontoxic metabolites). NOTE: AC doesnt work with alcohol poisonings |
|
|
|
Calculate PPV, NPV, Sn, Sp, PLR, NLR for a diagnostic test when a gold standard is available.
|
After setting up 2x2 table;
PPV = TP/(TP+FP) NPV = TN/(TN+FN) Sn = TP/(TP+FN) Sp = TN/(TN+FP) PLR = Sn/(1-Sp) NLR = (1-Sn)/Sp |
|
|
|
What causes DIC? How do you manage it?
|
Primarily due to an uncontrolled and excessive production of thrombin, leading to widespread and systemic intravascular fibrin deposition, and secondary bleeding diathesis due to consumptive coagulopathy.
Tx: Treat the underlying cause (commonly sepsis with endotoxemia, trauma, extensive surgery, or malignancy). Judicious replacement of clotting factors in bleeding patients, and occasionally use of heparin and/or APC, especially in sepsis. |
|
|
|
What are the clinical manifestations suggestive of HIT?
Name two laboratory tests you can order in suspected HIT? |
-Timing - thrombocytopenia occurring 5-10 days after the initiation of UFH (may occur earlier if recent heparin).
-Thrombocytopenia with platelet count dropping >50% from baseline, typically stays higher than 20,000, with a typical nadir around 50,000. -Thrombosis - both venous and arterial. -No other clear explanation. -Possible skin necrosis. Confirmatory laboratory tests: In addition to CBC, coag panel, can order: HIT serotonin release assay. Heparin induced platelet aggregation. Solid phase ELISA immunoassay. Note the first two are functional assays, the third is not. |
|
|
|
How do you treat HIT?
|
-Discontinue all heparin, including heparin-bonded catheters and heparin flushes. LMW heparin should also be avoided since it may crossreact with the heparin-induced antibodies
-Use of a direct thrombin inhibitor such as argatroban (hepatically cleared, good in renal failure), or bivalrudin/lepirudin in hepatic failure, or a heparinoid such as danaparoid. -Wait for plt higher than 150 before coumadin (risk of skin necrosis) |
|
|
|
What are the established indications for therapeutic hypothermia? What are some possible future applications?
|
Established - post cardiac arrest in the patient that has experienced ROSC, with global neurologic dysfunction (i.e. remain unresponsive).
Potential future applications: MI. Stroke. Traumatic brain injury. Acute hepatic dysfunction. |
|
|
|
What are the physiologic goals to be targeted in therapeutic hypothermia (i.e. degree of cooling, time to reach, etc.)?
|
-Cooling to 32-34 deg C, using cooling blankets and ice.
-Cool as quickly as possible - in the studies, 4-8 hours was required. -Eliminate shivering, using neuromuscular blockade and sedatives as necessary. -Duration of cooling 12-24 hours. -Rate of rewarming not well established; usually done via active rewarming with IV fluids and warm blankets. |
|
|
|
List risks associated with therapeutic hypothermia.
|
-Shivering - may work against goals of hypothermia, and increase metabolic rate and oxygen demand.
-Cold diuresis, and associated hypokalemia, hypomagnesemia, hypophosphatemia. -Increased risk of pneumonia, possibly secondary to immune dysfunction. -Increased risk of cardiac arrhythmia, particularly with temperatures lower than 32 C. -increased risk of acidosis and coagulopathy esp in trauma |
|
|
|
In a patient 1 month post colectomy, with a prosthetic mitral valve, on coumadin, what are possible causes of an acute drop in Hgb, other than GI bleeding?
|
1.Red cell underproduction (not acute)
-ACD -Bone marrow supression due to medications, sepsis, other intercurrent illness. -Nutritional failure, reduced iron absorption/delivery. 2. Red cell loss (acute) -Hemorrhage other than GI bleeding (eg. retroperitoneal secondary to line insertion attempts). -Phlebotomy. -Loss in extra-corporeal circuits. -Hemolysis - either immune mediated (auto-immune, drug induced, infection-related), or non-immune (mechanical on prosthetic valve, TTP/HUS, etc.) |
|
|
|
List non-infectious potential side effects from transfusion.
|
-TRALI.
-TACO. -Acute hemolytic transfusion reaction. -Delayed transfusion reaction. -Febrile transfusion reaction. -Urticarial transfusion reaction. |
|
|
|
In a patient involved in an MVA, struck from the left, what major chest injuries need to be considered?
|
Rib fractures, flail chest.
Pneumothorax, tension or non. Hemothorax. Pulmonary contusion. Cardiac contusion. Aortic disruption. Esophageal disruption. Diaphragmatic rupture. |
|
|
|
What are the CXR findings of aortic disruption?
|
Widened mediastinum.
Deviation of trachea to the right. First and second rib fractures. Loss of the aortic knob. Presence of a pleural cap. Depression of the left mainstem bronchus. Elevation and rightward shift of right mainstem bronchus. Obliteration of space between pulmonary artery and aorta. |
|
|
|
List the different characteristics of apoptosis vs. cellular necrosis.
|
Apoptosis/ Necrosis
Physiological or pathological /Always pathological Single cells/ Sheets of cells Energy dependent/ Energy independent Cell shrinkage/ Cell swelling Membrane integrity maintained /Membrane integrity lost Role for mitochondria and cytochrome C/ No role for mitochondria No leak of lysosomal enzymes /Leak of lysosomal enzymes Characteristic nuclear changes /Nuclei lost Apoptotic bodies form/ Do not form DNA cleavage/ No DNA cleavage Activation of specific proteases/ No activation Regulatable process/ Not regulated Evolutionarily conserved/ Not conserved Dead cells ingested by neighbouring cells/ Dead cells ingested by neutrophils and macrophages |
|
|
|
What are the cellular and subcellular pathophysiologic changes occurring in the spinal cord in a patient with traumatic SCI?
|
Apoptosis.
Cellular necrosis. Direct traumatic disruption. Cellular edema. Free radical release post reperfusion. Peri-injury penumbra, inflammation, inflammatory prostaglandins, IL-1, IL-6, thromboxane. |
|
|
|
What are the clinical characteristics of propofol infusion syndrome? Name the risk factors. What is the mechanism?
|
Clinical characteristics:
Metabolic acidosis. Cardiac failure - bradycardia. Rhabdomyolysis. Renal failure (myoglobinuria). Hyperkalemia. Diagnostic criteria: bradycardia has to be combined with lipaemic plasma, fatty liver enlargement, metabolic acidosis with base excess < -10 mmol/l, rhabdomyolysis or myoglobinuria. Risk factors: children prolonged administration (>48 hours) high dose (>5mg/kg/hr) glucocorticoids catecholamines closed head injury low energy supply Mechanism: uncoupling of respiratory chain results in mitochondrial dysfunction; increased free fatty acid production Other risks of propofol infusions: hypertriglyceridemia pancreatitis increased carbon dioxide production excessive caloric load |
|
|
|
Two coke-smokin’ crystal meth producers blow up their apartment. One develops an acute coronary syndrome, hypertensive crisis and seizes (once). His hypertension is refractory to benzodiazepines. What antihypertensives are contraindicated? If he is started on an infusion of Nipride and develops worsening seizures, worsening obtundation and ultimately refractory hypotension which does not resolve with discontinuation of the nitroprusside infusion, what should concern you?
|
Non-selective B-adrenergic antagonists contraindicated (may induce unopposed alpha adrenergic activity)
·Cyanide toxicity manifests as anxiety, dyspnea, headache, confusion, tachycardia, hypertension, stupor, coma, seizures, arrhythmias, cardiopulmonary collapse. ·Treatment (beyond supportive measures and oxygen) includes: amyl nitrite, sodium nitrite, sodium thiosulfate, hydroxycobalamin, dicobalt ethylenediamine tetraacetic acid (EDTA) |
|
|
|
A dope smokin' crystal meth producer whose lab exploded is completely comatose with refractory hypotension. A gung-ho clinical associate does a bedside echo and finds decreased myocardial contractility. After stating: “This is a GRRR-RREAT fellows’ case”, rubbing his head and then disappearing like a genie into a lamp, you notice the distinct charring of the face and body (c’mon, I’m trying to make this OBVIOUS!). The HbCO (!) comes back at 42%. List 3 mechanisms by which CO is a bad thing. If the patient is a woman (ha! Nowhere did I say she was a man!), baby-on-board should be protected by the greater affinity of foetal hemoglobin to oxygen, right? Gee, well how much greater is CO’s affinity for Hb than is O2? How much (how quickly) will O2 improve the situation?
|
·CO has a 240 fold greater affinity for Hb than does O2
·HbCO decreases O2 saturation proportionally to it’s concentration (HbCO of 50% decreases “effective” hemoglobin concentration by half ·CO toxicity results from: oLeft shift of oxyhemoglobin dissociation curve (decreased off-loading) oCarboxymyoglobin formation oBlockade of myoglobin-facilitated diffusion of oxygen, impairment of myoglobin-mediated oxidative phosphorylation resulting in decreased cardiac contractility oInhibition of enzymes in the mitochondrial electron-transfer chain oBinding and inhibiting Cyt P-450 and NADPH reductase ·Foetal hemoglobin binds CO to an even greater degree than adult Hb thus placing the foetus at higher risk than mom ·Breathing 100% O2 decreases the half-life of HbCO from 5-6 hours to 40 – 90 minutes. Adding 4.5-4.8% CO2 increases minute ventilation, maintains normocapnia and accelerates CO clearance (down to half-life of 31+/- 6 minutes) ·Hyperbaric oxygen (2.8ATM) decreases half-life to 15-30 minutes. |
|
|
|
A patient is rolled from supine to left lateral decubitus during the course of her ICU stay. Prior to rolling, both upper extremities give NIBP readings of 120/80. After rolling, her right arm reads 105/65, while her left arm reads 135/85. If the patient has both right and left radial arterial lines in situ, what will they now read respectively after turning, if they had good correspondence with the non-invasive cuff readings in the supine position?
|
·They will both read 120/80 because the pressure transducer has not moved.
|
|
|
|
What happens to vital capacity when passing from upright to supine position
a) For a quadriplegic patient at C5 b) For a patient with pure bilateral diaphragmatic paralysis |
a) C5 quad, diaphragms intact: Recumbency results in increase in the VC compared to the upright value; abdominal contents cannot force diaphragm up, and thus assist in expiration and inspiration.
b) In contrast, patients with bilateral diaphragmatic paralysis show a 50 percent decrease in the VC when they are supine. This is due to a cephalad displacement of abdominal contents without the normal concomitant increase in diaphragmatic tone. The associated alteration in ventilation-perfusion matching results in oxygen desaturation. |
|
|
|
In HFOV, name two strategies to treat rising CO2. How do they work?
|
1. Increase the amplitude (aka the power). Works by increasing the force with which the oscillatory piston moves, thereby increasing the volume of ventilation.
2. Decrease the frequency. Works by increasing the volume displacement and CO2 elimination. 3. Cuff deflation - may help by allowing CO2 to ventilate around the cuff. 4. Increase the i-time; allows for a larger volume delivery and increasing volume exchange (max i-time 50%). |
|
|
|
Name two possible side effects of permissive hypercapnea.
|
1. Increased intracranial pressure from intracranial hypercapnic vasodilation.
2. Potential myocardial suppression (usually short-lived). Caution in patients with myocardial dysfunction. 3. Increased pulmonary arterial pressures and pulmonary hypertension (? due to decreased nitric oxide). 4. Adverse fetal effects in pregnancy. 5. Need for sedatives or paralysis. 6. Post-hypercapnic metabolic alkalosis. 7. Blunted respiratory drive at extubation. |
|
|
|
How does a phonation valve work? Name 3 contraindications to its use.
|
One way valve; allows air entry on inspiration, closes on expiration allowing air to be exhaled over vocal cords and phonation allowed (needs cuff down trach).
Contraindications: Severe upper airway obstruction Tenacious pulmonary secretions Incompatibility with foam filled, cuffed tracheostomy tube Decreased cognitive status |
|
|
|
In a COPD patient on PSV who is clearly impossible to wean at day 5, name 4 advantages to perform tracheostomy.
|
Decreased patient anxiety.
Improved patient comfort. Increased ease of suctioning, secretion removal. Less need for patient sedation, allowing quicker wean. Easier, safer access to mouth; improves oral hygiene. Specially designed trachs allow for phonation, possibly eating. Reduction in anatomic deadspace, allowing greater alveolar ventilation for any minute ventilation. Easy to discontinue and reinstitute mechanical ventilation during weaning. |
|
|
|
In a patient with an acute exacerbation of COPD on PSV who appears uncomfortable, name 4 ventilator modifications that may alleviate the problem.
|
Increase level of pressure support to allow for increased Vt.
Change to a different mode (eg. volume control). Increase the PEEP to attempt to match auto-PEEP (goal 85% of intrinsic PEEP). Decrease the trigger level (i.e. make it easier to trigger a breath). Change to a different type of trigger (i.e. from flow trigger to volume trigger or vice versa). If significant auto-PEEP accounting for difficulty breathing, disconnecting from ventilator and allowing prolonged expiration may be of temporary benefit. |
|
|
|
What is the formula for oxygen delivery?
|
QO2 = Qt x CaO2
Where QO2 = oxygen delivery, Qt = total cardiac output, and CaO2 = the arterial content of oxygen. CaO2 = 1.34 x Hgb x SaO2 + 0.0031 x PaO2 |
|
|
|
What is the formula for oxygen extraction?
|
Extraction fraction = (CaO2-CvO2)/CaO2
Oxygen consumption = VO2 = Qt(CaO2-CvO2) Arteriovenous oxygen content difference = CaO2-CvO2 |
|
|
|
In a PEA arrest, what drugs and doses can/should be given? If no IV access is available, what alternate routes of drug delivery exist? What are the appropriate doses?
|
Epinephrine 1 mg IV q 3-5 minutes.
Atropine 1 mg IV if PEA rate is slow, repeated q 3-5 minutes, max dose 3 mg. If no IV access available, drugs can be given IO (same doses) or via ETT (double doses). |
|
|
|
In anaphylactic shock, what is the most important drug to give, and what is the dose? Name two other drugs and doses that can be given?
|
Epinephrine 1:1000 IM, 0.3-0.5 mg, or 1:10000 IV 0.3-0.5 mg.
Could also give: Benadryl (diphenhydramine) 25-50 mg IV/IM Ranitidine 50 mg IV Methylprednisolone 1-2 mg/kg IV, may reduce biphasic reaction. Levophed 5-20 mcg/min IV Glucagon if patient taking B-blockers (may be resistant to epi). 1-2 mg IV. Salbutamol for refractory bronchospasm (5 mg via neb prn). Obviously should also do all the non-pharmacologic stuff (IV/O2/Monitor/Fluids, etc). |
|
|
|
In a patient with Guillain-Barre syndrome, name two physical signs that may indicate the need for intubation.
Why might this patient not develop paradoxical breathing prior as a sign of impending respiratory failure? |
Inability to cough.
Inability to stand. Inability to lift the head or elbows. VC < 15-20 mL/kg. NIF < 20 cmH20 Paradoxical breathing may not be present, as patient may be too weak to generate enough negative intrathoracic pressure to raise diaphragms and cause abdominal indrawing. |
|
|
|
Give 2 side effects of neuroleptics and their respective treatment.
|
1. EPS (extrapyramidal symptoms) - eg dystonia, akathisia. Discontinue neuroleptic, trial of anticholingeric drug such as benztropine (cogentin). Alternatives include diphenhydramine, amantadine, benzodiazepines.
2. Neuroleptic malignant syndrome. Tx; discontinuation of offending agent, supportive care, dantrolene, bromocriptine, amantadine. |
|
|
|
Lithium overdose. 20 mg ingested. Level 6 hours post ingestion = 4 mmol/L
a) Give 4 acute complications b) What treatment is the most appropriate (name only 1)? |
Acute complications of Li overdose:
Neurologic: neuromuscular excitability, irregular coarse tremors, fascicular twitching, rigid motor agitation, muscle weakness, ataxia, sluggishness, delirium, stupor, coma, permanent neurologic sequelae (such as dementia and ataxia). CVS: sinus bradycardia, hypotension. GI: nausea, vomiting, diarrhea, Heme: leukocytosis Treatment: the most dialyzable toxin known, due to its low molecular weight, negligible protein binding, and volume of distribution similar to that of water. Thus, hemodialysis is the treatment of choice for severe lithium toxicity. |
|
|
|
Sympathomimetic toxidrome
a) Name 4 characteristics b) What best differentiates it from the anticholinergic toxidrome? |
Agitation, anxiety, mydriasis, diaphoresis, seizures, dry mouth, tachycardia, hypertension.
Best differentiated from anticholinergic toxidrome by dry skin, flushing, blurred vision. (Hot as a Hare, Dry as a Bone, Red as a Beet, Mad as a Hatter, Blind as a Bat) |
|
|
|
Define
a) First-order elimination b) Zero-order elimination c) Name how the following substances are eliminated 1- Acetominophen 2- Ethanol 3- Carboxyhemoglobin 4- Salicylate |
First order elimination (or first order kinetics) is defined as a constant FRACTION of drug being eliminated per unit time (logarithmic plotting of the concentration of the drug yields a linear graph).
Zero order elimination (or zero-order kinetics) occurs when a constant AMOUNT of drug, rather than a constant fraction, is eliminated per unit time. This can happen if elimination pathways become saturated. Small dose escalations can result in large plasma concentration increases. Ethanol, phenytoin and salicylates are protoypical examples of zero-order, capacity-limited clearance. The other drugs on the list - acetaminophen, carboxyhemoglobin, exhibit first order elimination. |
|
|
|
In a patient 24 hours post liver transplantation with climbing liver enzymes and INR:
Name 2 DDx. If you could only order one test, what would it be? |
DDx;
Hepatic artery or Portal vein thrombosis. Acute cellular rejection. Primary graft failure. Massive hemorrhagic necrosis Severe infection. First diagnostic test: Doppler ultrasound of hepatic vasculature to ensure good flow, and detect biliary tract abnormalities. |
|
|
|
Name 2 patient populations for which addition of glutamine to feeds may be beneficial.
|
Burns.
Trauma. Bone marrow transplantation. From latest Canadian guidelines (2009) "Based on 4 level 1 studies and 13 level 2 studies, when parenteral nutrition is prescribed to critically ill patients, parenteral supplementation with glutamine, where available, is strongly recommended. There are insufficient data to generate recommendations for intravenous glutamine in critically ill patients receiving enteral nutrition." |
|
|
|
Name 3 strategies to place a post-pyloric feeding tube.
|
Use of maxeran as a motility agent 10 mg IV 20 minutes prior to placement.
Use of erythromycin 250 mg 1/2 hour prior to placement to act as a motility agent. Use of laryngoscope or glidescope to confirm advancement of tube into the esophagus. Placement by frequent auscultation. Position patient in right lateral decubitus position. Flouroscopy. Endoscopy. Milking of tube distally during intra-abdominal surgery. |
|
|
|
Patient admitted for septic shock (pneumonia) on norepinephrine. NGT drains 500 ml.
a) When do you start to feed? b) Name 4 strategies to decr. risk of feeding complications. |
Start to feed as soon as patient is settled in ICU and stabilized from a hemodynamic perspective. Feeding within 24-48 hours of ICU admission is the recommendation.
Strategies to decrease the risk of feeding complications: Use of a feeding protocol. Use of post-pyloric feeding tubes. Use of metoclopramide as a promotility agent. Elevation of the HOB to 45 degrees. Tolerance of gastric residuals up to 250 mL. lower calorie goals if refeeding risk |
|
|
|
Name 4 non-pharmacologic ways (excluding transfusion of blood products) to treat a patient with recurrent variceal bleeding.
|
Pharmacologic:
Octreotide Pantoloc Antibiotics (cipr/ceftriaxone). Correct coagulopathy. B-blockade later. Non-pharmacologic: Endoscopic banding. Sclerotherapy. Minnesota/Blakemore-Sengstaken tube. Thrombectomy for portal vein or splenic vein thrombosis. TIPS. Porto-systemic shunt. Liver transplant. Distal spleno-renal shunt (DSRS) in case of thrombosed splenic vein causing gastric varices. Splenectomy in the case of splenic vein thrombosis causing gastric varices. Thanks to my surgical colleagues for the more interesting answers above; talk to them if you disagree. |
|
|
|
a)Name 4 criteria for hepato-renal syndrome
b) Name 2 treatments to improve renal perfusion |
Criteria:
Hepatic failure and portal hypertension. Acute rise in Cr > 133 umol/L. Absence of other causes of renal disfunction (shock, medications, obstruction, SBP). Lack of improvement with volume expansion (usually w/ albumin 1g/kg/day). No significant proteinuria, hematuria. 2 treatments to improve perfusion: Octreotide/midodrine Terlipressin Norepinephrine/albumin TIPS Liver transplant Dialysis (last three as a bridge to transplant). |
|
|
|
Regarding early goal directed therapy:
List 4 physiologic parameters that were assessed in the study, and their target values. |
CVP 8-12 in non-ventilated patient.
SBP >=90 MAP >= 65 U/O > 0.5 mL/kg/hr Hct > 30% MVO2 > 70% |
|
|
|
Name 2 pathogens for which you need an N95 mask.
|
Respiratory tuberculosis (mycobacterium tuberculosis).
Avian influenza Smallpox |
|
|
|
Clostridium difficile associated diarrhea (CDAD).
a) Name 4 risk factors to get CDAD. b) Why there is an outbreak of severe CDAD? c) Name 4 treatments for recurrent CDAD. |
Risk factors:
-Any antibiotic use, especially clindamycin, flouroquinolones, penicillin, cephalosporins. -Advanced age (>65) -Hospitalization/institutionalization. -Increased number of concurrently infected patients on same ward. -sharing a room with an infected patient. -Host immune suppression. -High endemic rates in the hospital/institution. -Poor infection control measures in the institution. -Possibly gastric acid suppression (controversial). Reason for outbreak of hypervirulent strain: -resistance to fluoroquinolones. -hypervirulence conferred by additional toxin production, and increased toxin producing capacity. Treatments for recurrent CDAD: -Metronidazole 500 mg po/IV q 8 hrs. -Vancomycin 125 mg po q 6hrs -Vancomycin retention enemas. -Cholestyramine (anion-binding resins) as adjunctive therapy. -Surgical resection (colectomy) in cases of toxic megacolon. -Fecal transplant in significant, frequent recurrences. |
|
|
|
Hypoglycemia
a) Give 2 medications causing hypoglycemia (except insulin) b) If patient has no IV access, what should you give? c) The patient has an IV, but also has recurrent hypoglycemic episodes. Name 2 medications to give to prevent recurrent hypoglycemia (and their associated mechanism). |
Medications causing hypoglycemia:
Glyburide Chlorpropamide (sulfonylureas) Quinolones (gatifloxacin) Pentamidine Quinine Alcohol Salicylates Betablockers ACEI Treatment without IV access: Glucagon 0.5 to 1.0 mg SC/IM Prevention of recurrent hypoglycemia: Somatostatin-inhibits insulin release Terbutaline Steroids Mechanism? |
|
|
|
Name 4 strategies to prevent radiocontrast nephropathy.
|
-Hydration with NS 1 mL/kg/hr for 12 hrs pre and post administration of contrast.
-Treatment with NaHCO3 3 mL/kg/hr x 1 hr pre and 1 ml/kg/hr for 6 hrs post administration. -Treatment with NAC 600 mg po q12 hrs pre and post. -Minimize unecessary administration of radiocontrast; consider non-contrast study as an alternative, or other modality (U/S, MRI, etc). -Use of non-ionic low-osmolal contrast agents. -D/C nephrotoxic drugs prior to contrast use (Lasix, NSAIDS, ACEI, ARB). |
|
|
|
Intoxication au FER chez un jeune de 23 ans
a. Donner le traitement initial b. Quel est le mécanisme thérapeutique en hémodialyse chez ce patient? c. Si pas d’amélioration après 6 heures de traitement, que faire? |
Initial treatment:
Supportive care. Volume resuscitation to maintain hypovolemia. Contact poison control. Consider GI decontamination if early, if systemic symptoms (AC for possible coingestion, GL). Deferoxamine if large amount of iron on flat plate, severe symptoms, high iron concentration >90, AGMA . PEG (whole bowel irrigation). Stages of intoxication; 1. GI symptoms. 2. Latent phase. 3. Shock secondary to hypovolemia, cardiac dysfunction, distributive shock. Mechanism of hemodialysis? Transport of circulating iron molecules bound to transferrin, needs to be initiated early prior to incorporation of iron into intracellular form. If no improvement after 6 hours -Rule out coingestion, other toxin. -Rule out retained pills and ongoing absorption in gut (bezoar) with x-ray. -If not given already, initiate deferoxamine and dialysis prn. Discuss with poison control. -Exchange transfusion as therapy of last resort. |
|
|
|
In a young patient post-op repair of an open fracture, who develops a violaceous rash, and pain at the operative site 12 hrs post, and a Hgb drop from 90 to 40, who presents in septic shock:
What is the most likely responsible organism? How do you explain the drop in Hgb - 2 possible causes: |
Most likely organism: Clostridium perfringens.
Cause of anemia: -intravascular hemolysis associated with bacteremia. -occult bleeding into the leg. |
|
|
|
Draw a CRRT circuit
|
Make sure you have a filter, with an inbound line for venous inflow, and an outbound line for arterial outflow.
Show countercurrent dialysate inflow and outflow. Indicate pre-filter replacement fluid and post-filter replacement fluid. |
|
|
|
In a patient with extensive burns, list three causes of fluid resuscitation requirements being greater than that predicted by the Parkland formula.
|
The Parkland formula (4ml/kg/hr/%TBSA 2nd and 3rd degree burns, half in first 8 hours, remainder in 16 hrs) commonly underestimates fluid requirements in:
-inhalational injury -high voltage electrical injury -delayed resuscitation -massive deep burns -circumferential burns with compartment syndrome -rhabdomyolysis -associated traumatic injury -pre-existing dehydration -hyperglycemia -alcohol intoxication -chronic diuretic therapy |
|
|
|
List the components of the TIMI risk factor score for UA/NSTEMI
|
Age>65
>3 CAD risk factors Prior CAD ASA in last 7 days >2 anginal events in last 24 hrs ST deviation Elevated cardiac markers |
|
|
|
Given a mechanically ventilated patient whose breathing is dysnchronous with each mechanically delivered breath, list 5 ventilator factors affecting synchrony.
|
-Trigger sensitivity
-Initial flow delivery -ability to alter flow -inspiratory time -tidal volume -PEEP, auto-PEEP |
|
|
|
Name 4 principles for quality improvment, as espoused by the Institute for Healthcare Improvement.
|
Establish answers to the following:
What are we trying to accomplish? Aim statement. How will we know that change is an improvement? Establish measures. What changes can we make that will result in an improvement? Decide which changes are most likely to create improvement ("All improvement requires change, but not all change is an improvement"). Test changes in the working environment via PDSA (plan, do, study, act) cycles. |
|
|
|
In an electrical injury:
Name 3 major complications (excluding death) from electrical injury. Name the most common ocular and most common otic manifestations of lightning strike injury. |
Complications:
-Arrhythmia -Deep tissue burns -Shock from myocardial dysfunction, hypovolemia 2ndary burns. -Blunt mechanical injury from lightning strike, muscle contraction, or as a complication of a fall after electrocution. -loss of consciousness -weakness or paralysis (keraunoparalysis) -respiratory depression -memory disturbances -Autonomic dysfunction (including hypotension, bradycardia, dilated or asymmetric pupils). -Rhabdomyolysis -Ruptured TM -Cataracts |
|
|
|
Name 2 modifications to the ACLS protocol if a patient is in VF, with a body temp of 29 C.
|
1. Shock once, but if no cardioversion, continue warming and try again after temp > 30 C.
2. Withold medications until temp > 30 C, then consider reduced frequency of dosing to avoid accumulation with reduced circulation. 3. Consider more frequent changes of CPR providers, as chest wall may be stiff, and CPR more difficult. 4. Increased focus on active rewarming as opposed to rhythm correction and ROSC. |
|
|
|
18 yo girl, hit by motorcycle, abdominal pain, grade I , no other abnormalities. Liver panel, CBC, coags normal. Some hours later, deteriorates with shock and abdominal distention.
a. 2 DDx. b. 2 investigations that will help distinguish one DDx from the other. |
DDx;
Splenic rupture, bleeding from other source (spleen, liver, pancreas). Traumatic pancreatitis. Perforated viscous, peritonitis. Investigations: Abdominal U/S (FASST), Repeat CBC, amylase, lipase, repeat CT, laparotomy. |
|
|
|
Narrow complex tachycardia in a patient post cardiac surgery with epicardial wires in situ. You have performed vagal maneouvers and given adenosine. Post adenosine ECG show ongoing flutter waves, with no QRS complexes.
Give 2 non-pharmacologic maneouvers to treat the patient. |
Appears to be complete heart block - ? cause - unlikely to be adenosine.
Anyway, if you repeat the ECG and confirm complete AV block, could go ahead and: a) initiate ventricular pacing via the epicardial wires. b) initiate CPR while pacing is arranged. c) attempt synchronized cardioversion of the a-flutter d) consider overdrive atrial pacing of the a-flutter (ensure you use the right sided leads, and that there are atrial leads in situ to avoid overdrive pacing the ventricles). |
|
|
|
30 yo patient, presents with odynophagia, cervical neck pain. CT scan reveals inflammatory changes around the vocal cords (sic?), bilateral pleural effusions and pneumomediastinum.
Read the ECG; what is the primary finding? (Shows diffuse ST elevation and PR depression). What is your primary diagnosis and treatment? |
ECG finding consistent with pericardial inflammation.
Provisional diagnosis; posterior pharyngeal abscess with mediastinal spread. DDx includes esophageal erosion 2ndary to infection. Tx: Supportive care, correction of shock, EGD therapy if active sepsis. Abx: clindamycin and gram negative coverage (eg. gent, third gen ceph), or more likely imipenem or pip-tazo. Consider addition of Vanco in susceptible/at risk host. Source control; drain abscess, effusions, consider mediastinoscopy if evidence of infection, investigate esophagus for signs of perforation and surgical repair prn, drain pericardial effusion prn. |
|
|
|
CXR shows the tip of a "jugular" CVC projecting towards the aortic valve.
Two steps you would take. |
1. Confirm arterial placement vs. persistent left SVC (1% of population; drains into coronary sinus):
-Hook catheter to pressure transducer to confirm arterial waveform and pressures. -Send blood gas from catheter; confirm arterial blood gas values. -echo shows enlarged coronary sinus in persistent left SVC 2. Arrange for correction of the problem, if arterial. -Discuss situation with vascular surgeon; do not remove catheter until discussed with them. -Correct any coagulopathy prior to either surgical or bedside catheter removal. 3. If not arterial, and in persistent left SVC, can leave alone and use. |
|
|
|
Name two clinical or biochemical elements that distinguish the PTT from the INR.
What is the primary treatment of a prolonged PTT? |
PT/INR measures the extrinsic pathway (i.e.the TF pathway) and the common pathway.
PTT measures the intrinsic pathway (i.e. the contact activation pathway) and the common pathway. INR is prolonged with coumadin administration. PTT is prolonged with heparin administration. Therefore primary treatment of prolonged PTT is heparin discontinuation. Any others? Feel free to edit this card. (Translated poorly from the French). |
|
|
|
40 yo previously healthy patient, presents with first episode seizures, treated with Lorazepam and Dilantin. Intubated, ventilated.
48 hrs later, still comatose despite discontinuation of sedatives. MRI/CT normal. LP reveals lymphocytic pleocytosis, elevated protein, a few red cells. Acyclovir is started on the suspicion of herpetic encephalitis. A) The patient is afebrile despite the CSF leucocytosis, and stable hemodynamically. Neuro exam reveals no focal abnormalities. What is the most likely diagnosis to explain his state? What is the treatment to initiate? |
Dx: nonconvulsive status epilepticus.
Tx: If it has been D/C'ed, reinstitute dilantin Tx (consider repeat load, daily dosing). May consider addition of Keppra if still seizing on dilantin. Midaz infusion. Add propofol if ongoing seizures on EEG. Either continuous or frequent serial EEG monitoring. Consider addition of ceftriaxone/vancomycin +/- ampicillin pending CSF gram stain and culture results. Don't forget to send CSF for TB PCR. |
|
|
|
Pt. post high velocity trauma, in respiratory distress, has two chest tubes in situ. CXR and CT show large tension PTx.
Question states: a)Give 4 abnormalities on the CXR or CT. b)strategies to minimize the leak. c)4 strategies to minimize morbidity and morality. |
a) CXR findings:
-air in pleural space. -shift of trachea away from side of tension. -mediastinal shift away from side of tension. -chest tubes, other lines, etc., and their placement. -pulmonary collapse second to Ptx. b) strategies to minimize the leak. -if possible continue spontaneous ventilation (as opposed to positive pressure ventilation). -ensure current chest tube is patent and draining. -consider addition of further chest tubes. -if on PPV, minimize tidal volumes, minimize PEEP; permissive hypercapnea; may need to sedate to institute. -independant lung ventilation with minimal volumes on side of leak, good lung down to optimize V/Q matching. -thoracic surgical consult re: surgical repair of leak, glue injection, lung resection, etc. -bronchial occlusion with balloon tipped catheter. -consider jet ventilation/HFOV. -consider ECMO. Strategies to minimize morbidity/mortality in this patient in ICU? -Tertiary survey. -Daily multidisciplinary rounds. -Closed unit, managed by intensivists. -HOB elevated to 45 deg. -RT driven weaning protocol. -Daily spontaneous breathing trial. -Minimize sedation - consider daily wakening. |
|
|
|
Previously health young man, 12 days post trauma. Asymmetric chest auscultation and dyspnea, insertion of a chest drain into pleural effusion reveals milky liquid drainage.
What is the provisional diagnosis? What are two lab tests that will confirm your diagnosis? What is the medical treatment (not surgical)? |
-Dx: chylothorax secondary to thoracic duct injury.
-Confirmatory lab test: triglycerides and chylomicrons in pleural drainage. -Other tests: cell count and differential, pH, cholesterol, glucose, lactic dehydrogenase (LDH), total protein, cytology, and microbiologic smear and culture. -pleural fluid to serum glucose ratio assists in differentiating a chylous effusion (ratio <1) from extravasation of lipid and glucose-containing parenteral nutrition (ratio >1) -Treatment: -chest tube placement -maintenance of fluid and electrolyte balance -bowel rest with parenteral nutrition to minimize the flow of thoracic duct chyle -if ongoing high flows, surgical ligation +/- repair. -persistent high flows (>14 days) leads to immune suppression. |
|
|
|
Give 4 methods to minimize mortality and decrease ICU length of stay (do not give therapeutic maneouvers).
|
1. Daily multidisciplinary rounds.
2. Closed unit, intensivist run. 3. Minimize sedation, consider daily waking trials. 4. RT driven ventilator weaning protocol. 5. Daily SBT for all patients. 6. Maintain HOB at 45 degrees. |
|
|
|
Name two predictors of poor neurologic outcome in first 24 hours for a comatose patient post cardiac arrest.
|
1. Absent pupillary response to light, or other brainstem reflexes.
2. Myoclonic status epilepticus. 3. Abscence of N20 somatosensory evoked potentials. 4. Elevated levels of neuron specific enolase. |
|
|
|
How do you explain persistent vegetative state in lay terms?
|
Primitive brain function present, but no higher brain function, including thinking, emotions, or awareness, ability to experience any senses. No chance of ever having return of previous neurologic function.
|
|
|
|
Name four deficits found at 6 months in ARDS survivors.
|
1. Myopathy.
2. PTSD. 3. Poor performance on 6 minute walk test. 4. Poor results for quality-of-life measures. 5. Mild impairments in pulmonary function. 6.carbon monoxide diffusion capacity remained low |
|
|
|
How does heliox work? What are the disadvantages of using it?
|
Low density does two things:
1. decreases pressure gradient required for flow. 2. favors conversion of turbulent flow to laminar flow. Disadvantages: -Expense. -May mask worsening airflow obstruction, so less time or margin for error if moving from facemask to intubation. -Controversial data supporting its efficacy in non-intubated status asthmaticus. -Does not treat bronchospasm or airway wall inflammation. -High, squeaky voice. |
|
|
|
In subarachnoid hemmorrhage:
a) name 3 predictors of vasospasm. b) what is triple H therapy? How does it work? |
a)Predictors of vasospasm:
-time post SAH; days 5-10. -Hunt-Hess grade III-V (severe neurologic symptoms assoc. with SAH). -Fisher grade 3 (localized SA blood, or layers of blood > 1mm thick). -hypovolemia. -hypotension. Triple H therapy: Hemodilution, hypervolemic, hypertensive Tx to maintain cerebral perfusion. Optimal hydration status; whatever is necessary to optimize Qt (CO). MAP goal 15-20% above baseline initially, then raise higher if ischemic deficit does not clear in 1-2 hours, to a max of 150-160. Continue tx 3-4 days prior to attempt to wean gradually. Need close monitoring to watch for fluid overload, electrolyte imbalances. |
|
|
|
Name 4 classes of drugs that can cause QT prolongation. Give examples of each
|
1. Antibiotics:
Flouroquinolones: moxifloxacin, ciprofloxacin. Macrolides: erythromycin, clarithromycin 2. Antipsychotics: haloperidol, risperidone, methotrimeprazine. 3. Antidepressants: TCAs, SSRIs 4. Narcotics: Methadone 5. Antiarrhythmics: Sotalol, amiodarone, procainamide 6. Antihistamines: terfenadine |
|
|
|
List 3 physiologic changes of the airway that can lead to difficulty in airway management in pregnancy.
|
-Upper airway edema.
-Diminished airway caliber (use smaller tube). -Friability and propensity for hemorrhage. -Increased risk of aspiration due to decreased lower esophageal sphincter tone. -Rapid desaturation with apnea due to decreased FRC and lower pulmonary reserve. -Aorto-caval compression causing decreased preload and propensity for hypotension. -Enlarged breasts can cause difficulty in using laryngoscope due to obstruction of handle. |
|
|
|
Name 4 potential complications to mother and/or fetus from blunt abdominal trauma.
|
Fetal demise.
Initiation of preterm labor (25%). Uterine rupture. Abruptio placenta. Isoimmunization. Increased risk of hemorrhage, as blood flow to entire pelvis increased. Amniotic membrane rupture. |
|
|
|
List 4 predictors of failure after 2 hours of NIPPV.
|
Decreasing oxygenation (increasing FiO2 requirements).
Increasing PaCO2, decreasing pH. Poor compliance. Significant air leak. Altered LOC. Copious secretions. Pt./ventilator asynchrony. |
|
|
|
Regarding RBC storage, describe 2 storage effects and their consequences.
|
1. Decreased 2,3-DPG causes shift of oxyhemoglobin dissociation curve to the left, and decreases O2 offloading at the tissues, leading to decreased tissue oxygenation.
2. Decreased deformability of red cells leads to more rapid cellular destruction, and decreased half life. 3. K+ release during storage can lead to relatively high levels of K+ in transfused units, and therefore higher levels in patients. 4. ATP depletion causes increased osmotic fragility and decreased half life. Red cells are customarily stored for 72-90 days. Longer than that increases the chance of bacterial transmission. |
|
|
|
Name 5 blood conservation strategies that can be utilized to decrease transfusion needs of an ICU.
|
1. Conservative transfusion threshold (eg. Hgb<=70) in appropriate patients.
2. Reduce or eliminate routine daily bloodwork. 3. Point of care and inline bedside microanalytic techniques. 4. Transfusion of single units of blood at a time. 5. Health worker education programs. 6. Use of recombinant human erythropoeitin. 7. Intraoperative scavenging and reuse of red cells via cell saver techniques. |
|
|
|
In a patient with an air embolus, what is your immediate management? Name 2 other interventions to correct the situation. What changes might you see on an end-tidal CO2 detector?
|
Immediate management:
-Identify and close source of air , if possible (esp. look at CVCs). -Place the patient in Trendelenburg (head down) position, and left lateral decubitus (right side up), in an attempt to keep air in R ventricle. -100% oxygen to enhance nitrogen washout. -Fluid bolus to fill RV, decrease gradient for air to enter venous system. -Lower airway pressures (decrease PEEP, tidal volumes). -CPR if no cardiac output. Further treatment: -reduce cardiac filling pressures to lowest level that allows adequate oxygen delivery, to minimize non-cardiogenic high permeability pulmonary edema (i.e. ARDS) from inflammatory mediators. -Consider attempt at aspirating air via CVC, possible PAC, direct echo guided aspiration, or surgical removal via thoracotamy. -steroids may reduce inflammation causing lung injury. -Hyperbaric O2 can be of assistance if patient stable, or if R sided air embolus has become systemic via PFO or intra-pulmonary AVM. End-tidal CO2 detector will show decreased CO2, due to decreased perfusion of lung units, V/Q mismatching, increased Vd/Vt, and decreased gas exchange. BTW, did you know a cause of air embolism (according to Hall et al.) is orogenital sex? Makes you think, doesn't it? |
|
|
|
List 1 item from history and 1 from physical exam that would suggest malnutrition on a Subjective Global Assessment
|

A. History
1. Weight Change Maximum weight________ Wt 1 year ago______ Wt 6months ago_____ Current Wt__________ Overall loss in past 6 months: amount = #__________lbs; % loss = __________. Change in past 2 weeks: __________increase, __________no change, __________decrease. Other history: (Change in clothing size, loose fitting clothes....) A=No significant change; B=5-10% weight loss; C= 10% or more sustained weight loss 2. Dietary intake change (relative to normal) (Have eating patterns changed over last weeks or months? Has amount of food eaten changed? Are certain foods they used to eat that they no longer eat? What happens if they try to eat more? How does typical breakfast, lunch, dinner compare with six to twelve months ago?) A=No significant change; B=poor but improving or borderline but declining; C=starvation, unable to eat 3. Gastrointestinal symptoms (that persisted for > 2 weeks) ___none (A), ___Some symptoms (B) (nausea, vomiting, diarrhea, anorexia. ___Many symptoms (C) 4. Functional capacity ____No dysfunction (e.g., full capacity),(A) ____Dysfunction : mild (B); ____Severe (C) __________duration = #__________weeks. 5. Disease and its relation to nutritional requirements Metabolic demand (stress): __________no stress (A), __________low-moderate stress (B), __________high stress (C) B. Physical (for each trait specify: A = normal, B = mild-moderate, C = severe). # __________loss of subcutaneous fat (triceps, chest) # __________muscle wasting (quadriceps, deltoids) # __________ankle edema # __________sacral edema # __________ascites C. SGA rating (select one) __________A = Well nourished __________B = Moderately (or suspected of being) malnourished __________C = Severely malnourished |
|
|
|
List risk factors/mechanisms of injury that suggest possible blunt vascular neck injury.
Management? |
Head injury with GCS <=8.
Major thorax injury. Basal skull fracture. Facial injury (lefort 2 or 3). Other neck injury. Management: -Aspirin. -Referral to interventional radiology for possible stenting. "Blunt vascular neck injuries: diagnosis and outcomes of extracranial vessel injury. McKevitt EC, Kirkpatrick AW, Vertesi L, Granger R, Simons RK. Department of Surgery, Vancouver General Hospital, University of British Columbia, Canada. J Trauma. 2002 Sep;53(3):472-6." |
|
|
|
How do you clinically differentiate CSW from SIADH?
|
"CSW mimics all of the laboratory findings in the SIADH. The only clue to the presence of CSW rather than SIADH is clinical evidence of extracellular volume depletion, such as hypotension and decreased skin turgor, and/or increased hematocrit, in a patient with a urine sodium concentration above 40 meq/L. Unlike SIADH, volume repletion in CSW leads to a dilute urine, due to removal of the hypovolemic stimulus to ADH release, and subsequent correction of the hyponatremia. This finding has not been convincingly demonstrated which could reflect concurrent SIADH due to the CNS disease.
In patients with a clinical picture compatible with CSW, we recommend initial therapy with isotonic saline to correct the volume depletion and possibly reverse the hyponatremia." Reference: uptodate |
|
|
|
On a concentration/time graph of antibiotic dosing, label the concentration above MIC, the AUC above MIC, and the time above MIC.
|
See image.
|
|
|
|
For an antibiotic relying on concentration dependant killing, what pharmacokinetic/pharmacodynamic parameters correlate best with clinical efficacy.
How about for an antibiotic exhibiting time dependent killing kinetics? List examples of antibiotic that primarily depends on each of the PK/PD parameters you listed above. |
Concentration dependent killing; depends on Cpeak/MIC and/or AUC above MIC/MIC.
Time dependent killing depends on %time > MIC. Concentration dependent killers: Aminoglycosides depend on Cpeak/MIC. Fluouroquinolones depend on AUC/MIC Time dependent killers: beta-lactams, vancomycin depend on % time > MIC. |
|
|
|
If a bacteria susceptible to an aminoglycoside (eg. tobramycin) has an MIC of 2.0 mg/L, what would be an optimal Cpeak concentration for tobramycin in that patient?
If the patient's volume of distribution is 24 L, what once-daily dose of tobramycin would be appropriate? |
Optimal peak concentrations are anywhere from 6-20 x MIC. If you choose 10 x MIC as your peak conc. goal, the optimal concentration in this case would be 20 mg/L.
If Vd is 24 L, then to achieve 20 mg/L, you need 20x24 mg, or 480 mg/dose. |
|
|
|
If you need to reduce the dose of ciprofloxacin and ceftazidime in a patient due to decreasing Cr clearance, what changes to the dosing regimens would you recommend. What is your rationale?
|
Ciprofloxacin is a concentration dependent killer. Therefore need to achieve same concentrations; if dose is to be reduced, recommend same dose, increased interval.
Ceftazidime is a time dependent killer. Therefore, needs to have a significant time above MIC to be effective. Therefore, if dose needs to be decreased due to decreased clearance, maintain same interval, but decrease dose. |
|
|
|
List 4 differences in management of venous thromboembolism in pregnant patients relative to non-pregnant patients.
|
-Coumadin contraindicated.
-Thrombolytic therapy contraindicated at term, relatively contraindicated throughout. -IVC filter placed suprarenally. -Leg doppler U/S less reliable. -Doses of prophylactic heparin higher than 5,000 U SC bid may be required. -CT scanning should be minimized due to radiation risks to fetus. |
|
|
|
According to the most recent ASPEN guidelines, list three patient populations that should be placed on immune modulating enteral formulations (supplemented with agents, such as arginine, glutamine, nucleic acid, omega-3 fatty acids, and antioxidants)
|
-major elective surgery
-trauma -burns -head and neck cancer -critically ill patients on mechanical ventilation -"being cautious in patients with severe sepsis" |
|
|
|
According to the latest ASPEN guidelines, list three patient populations that may benefit from the addition of probiotics to feeds.
|
-transplantation
-major abdominal surgery -severe trauma note: may increase mortality in pancreatitis |
|
|
|
According to ASPEN guidelines, list three methods to improve EN tolerance in patients with severe acute pancreatitis.
|
1. Early enteral nutrition, to minimize the ileus associated with delayed feeding.
2. Small bowel (post-pyloric) feeding tube. 3. Switching from bolus to continuous infusion. 4.Nearly fat-free elemental feeds. |
|
|
|
In a post-surgical patient with decreased urine output and intra-abdominal pressure of 28:
What is your first step in management? Briefly describe how bladder pressure is measured. List three patient factors that may affect the accuracy of bladder pressure measurement. |
Initial management:
-Ongoing fluid resuscitation, but avoid excessive resusc. -sedate, possible paralysis -lay flat -drain acites/abscess -NG tube to drain stomach contents. -Consult with surgery re: abdominal decompression. IAP measurements should be: 1. Expressed in mmHg (1 mmHg = 1.36 cm H2O) 2. Measured at end-expiration 3. Performed in the supine position 4. Zeroed at the iliac crest in the mid-axillary line 5. Performed with an instillation volume of no greater than 25 mL of saline [1 mL/kg for children up to 20 kg] (for bladder technique) 6. Measured 30-60 seconds after instillation to allow for bladder detrusor muscle relaxation (for bladder technique) 7. Measured in the absence of active abdominal muscle contractions IAP measurements may be inaccurate in : -Presence of dense intraperitoneal adhesions. -Pelvic hematoma. -Abdominal packing. -Neurogenic bladder. |
|
|
|
Why does jaundice occur with acalculous cholecystitis?
How do you manage AC? |
Secondary to partial biliary obstruction from inflammation extending in to CBD.
Management: Antibiotics. Cholecystectomy, or percutaneous drainage of abcess. |
|
|
|
Define ARDS.
What are the clinical criteria that have been used in research studies to identify ARDS? |
Non-hydrostatic, permeability pulmonary edema.
Criteria: -Acute onset. -Bilateral infiltrates on CXR. -FiO2/PaO2 < 200 (less than 300 for ALI) -No evidence of LV failure (PAOP < 18 mm Hg) -Some say decreased compliance (Cst < 40 mL/cmH2O). |
|
|
|
In a patient presenting with symptoms of myxedema coma, name two lab tests you should send before starting treatment.
What treatment should you start? |
Check TSH, free T4, cortisol.
Treatment: Thyroid hormone administration (T3, T4, or both). Hydrocortisone. Supportive care. Empiric broadspectrum Abx until infection ruled out. |
|
|
|
In using arterial pulse pressure variation to assess fluid responsiveness, what gives the most useful information for resuscitation - the delta down, delta up, or the total systolic pressure variation?
With respect to the patient's status, give one requirement to make the concept valid (AKA what am I thinking). |
Delta down.
Patient cannot be triggering the ventilator; only works during controlled ventilation. |
|
|
|
List three phases of damage control strategy in severe traumatic intra-abdominal injuries.
List three salient intraoperative patient factors to implement damage control. |
Phases:
1. pt selection (not everyone needs it) 2. operative hemmorrhage and contamination control and temporary closure 3. restore physiology in ICU Intraoperative factors: -coagulopathic -hypothermic -acidotic -multiple injuries -anticipated need for time consuming procedure -inaccessible major vascular injury (eg retrohepatic cava) |
|
|
|
List four findings on CT scans that help to predict outcome in traumatic brain injury.
|
-Degree of midline shift
-Presence or abscence of lesion densities. -Size of lesion densities. -Degree of ventricular compression. |
|
|
|
List 5 secondary brain insults following traumatic brain injury that are associated with increased mortality.
|
-hypotension (SBP < 90 mmHg)
-hypoxemia (sats < 90, paO2 < 55) -prolonged hypocapnia (PaCO2 < 30) -hypoglycemia -hyperthermia (temp. > 39C) |
|
|
|
Five signs of RV failure on CT scan in massive PE.
|
Dilated RV
Septal shift. RV sharing apex with LV. Dilated RA. Dilated SVC, IVC, azygous. Contrast reflux into liver, azygous. Contrast through open PFO. |
|
|
|
Name two potential complications of administration of carbohydrates as the sole source of non-protein calories (i.e. if you are withholding lipids in TPN).
|
-Fatty liver.
-Increased RQ, increased CO2 production. -Lipogenesis. |
|
|
|
When using an esophago-gastric balloon to tamponade variceal bleeding:
List two complications. List one key step to ensure tube is not inflated in the esophagus (assuming you use the gastric balloon pressure monitoring technique). What is the total amount of air used to inflate the gastric balloon? What is the target esophageal balloon pressure, and why? |
Complications:
-Esophageal erosion. -Esophageal perforation. -Aspiration. -Airway compromise. Key step: -Ensure you inflate the balloon OUTSIDE the patient, and check the pressure. Then, when inflating the balloon in the patient, inflation pressure should be within 5 cm H20 of the same pressure. Total amount of air: 250-300 cc. Target esophageal balloon pressure: 20-40 mmHg; need to achieve higher pressures than the portal vein. |
|
|
|
Two indications for TIPS?
What is the optimal target hepatic-venous pressure gradient to be achieved after TIPS to prevent further variceal bleeding? |
Indications:
-Refractory variceal bleeding. -Refractory ascites. Target gradient: 12 mmHg. |
|
|
|
List the three key lab tests in the MELD score. What is it used for?
List five key elements in the Child-Pugh score. What is it used for? |
MELD for liver transplant candidacy uses:
-Bilirubin -Creatinine -INR Child-Pugh to assess the severity of liver disease uses: -Bilirubin -INR -Albumin -Encephalopathy presence/absence and response to treatment. -Ascites presence/absence and response to Tx. |
|

