![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
Bronsted-lowry acid |
A proton (H+) donor |
|
|
Bronsted-lowry base |
A proton (H+) acceptor |
|
|
Alkali |
A base that dissolves in water forming OH- ions |
|
|
Neutralisation |
A chemical reaction in which an acid and base react together to produce a salt and water |
|
|
Acid+carbonate= |
Salt+water+carbon dioxide |
|
|
Acid+base= |
Salt+water |
|
|
Acid+alkali= |
Salt+water |
|
|
Acid+metal= |
Salt+hydrogen |
|
|
Acid-base pair |
Is a pair of two species that transform into each other by gain or loss of a proton |
|
|
Acid dissociation of HNO3 and HCOOH |
HNO3 -> NO3- + H+ HCOOH《=》HCOO- + H+ |
|
|
Why use a pH scale? |
As its a more conveniant way of measuring concentration of H+ |
|
|
Low pH value= High pH value= |
High H+ concentration Low H+ concentration |
|
|
[H+]= pH= |

|
|
|
Strong acids |

|
|
|
Weak acids |

|
|
|
Strong Acid definition and working out pH |

An acid that fully dissociates H+ = A- as fully dissociates So see diagram
|
|
|
Weak acids |

An acid that partially dissociates [H+] doesnt = [A-] So see diagram |
|
|
Acid dissociation constant |

|
|
|
Ka and pKa |

Large Ka means large dissociation so strong acid Small Ka means small dissociation so weak acid |
|
|
Ionic product of water |

|
|
|
Significance of Kw |
Kw controls the balance between [H+] and [OH-] In H2O [H+]=[OH-] In acids [H+] > [OH-] In alkalis [H+]<[OH-] |
|
|
Strong bases |
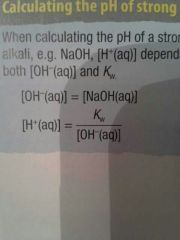
OH=KOH as fully dissociates Then see diagram and then pH=-log(H+) |
|
|
Buffer solution |
A mixture that minimises pH changes on addition of small amounts of acid or base |
|
|
Whats a buffer solution made up of? |
A weak acid and the salt of the weak acid |
|
|
2 ways to make a buffer solution? |
1) mix an acid and the salt of the acid 2) or partially neutralise a weak acid by an alkali |
|
|
Buffers |

Then pH=-log(H+) |

