![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back

Identify this piece of equipment.
|
Fire extinguisher
|
|

Identify this piece of equipment.
|
Fire blanket
|
|

Identify this piece of equipment
|
Eye wash
|
|
|
List the three pieces of emergency safety equipment.
|
Fire extinguisher
Eye wash Fire blanket |
|

What do you use an apron for?
|
To protect your clothing
|
|
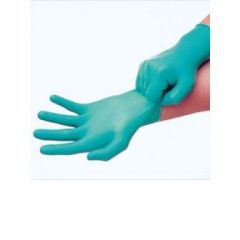
What do you use gloves for?
|
To protect your hands and keep chemicals from your hands out of your experiment
|
|
|
Why is washing your hands important in a lab?
|
To keep germs off your hands and out of your experiment
|
|

What is this piece of equipment?
|
Safety goggles
|
|
|
List the three pieces of preventative safety equipment.
|
apron
gloves goggles |
|
|
What is the formula for density?
|
D = mass / volume
|
|
|
Matter
|
anything that takes up space and has mass
|
|
|
volume
|
the amount of space taken up, or occupied by an object
|
|
|
What is the formula for volume of a regular solid?
|
Volume = length x width x height
|
|
|
What is the formula for volume of an irregular solid?
|
Volume = volume (water) - volume (water + object)
|
|
|
mass
|
the amount of matter that something is made of
|
|
|
Units for mass
|
grams
|
|
|
Malleable
(Malleability) |
A material that can be hammered into a flat sheet
|
|
|
Ductile
(Ductility) |
A material that can be pulled into a long wire
|
|
|
Luster
|
Another term for shine/shiny
|
|
|
Physical Property
|
A characteristic of a substance that can be observed without changing the substance into another substance (hard, brittle, soft)
|
|
|
Conductivity
|
The ability of an object to transfer heat or electricity to another object
|
|
|
Metals
|
These are often shiny, malleable, ductile, and they conduct electricity, and are magnetic
|
|
|
Nonmetals
|
These are dull, brittle, break easily, and are poor conductors of electricity
|
|
|
Metalloids
|
These can be solid, brittle, hard, and can conduct electricity
|

