![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
94 Cards in this Set
- Front
- Back
|
what is mononucleosis characterized by? |
an increase in mononuclear leukocytes
sx: fatigue, fever, sore throat, lymphadenopathy |
|
|
what is the etiologic agent for mononucleosis? what does it have an affinity for? |
EBV (Epstein-Barr Virus) (dsDNA herpes virus, spread by oral contact)
w/ an affinity for B-lymphocytes |
|
|
what would you see on a CBC differential of someone diagnosed w/ mononucleosis? |
increase in lymphocytes (10-20% reactive/atypical T lymphocytes*)
(NOT monocytes) |
|
|
what does the reactive (atypical) lymph in infectious mononucleosis look like? |
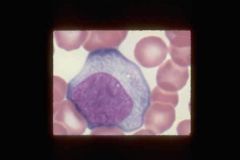
-t-cells w/ abundant cytoplasm; -chromatin not as dense as resting lymphocyte. -Blueing around the edges of the cytoplasm (blue skirt effect) -hugging the RBCs |
|
|
what two types of lymphocytes can you see on blood smear that is indicative of mononucleosis? |
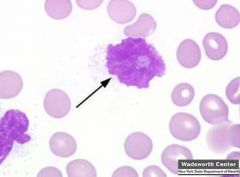
reactive T lymphs & degenerated B lymph (smudge cells)(arrow) |
|
|
How does EBV (infectious mononucleosis) cause infection? |
EBV attaches to epithelium (pharyngitis)--> gets into lymph & blood stream--> infects B cells--> Some B cells become activated plasma cells, while others remain latent--> infected plasma cells produce normal Abs & heterophile Abs--> T cells respond to infection--> T cells lyse infected plasma cells, latent cell become immortal (T cells do not attack) |
|
|
Infected plasma cells produce, heterophile, EBV, & autoantibodies. What are the EBV antibodies associated with infectious mononucleosis? (4) |
EBV-VCA (IgM), EBV-VCA (IgG), EBNA, EBV-EA |
|
|
Heterophile Abs react w/ Ags from different species, including bovine erythrocytes but NOT ______________ This is the basis for what test? |
NOT to guinea pig kidney cells
Monospot (rapid slide differential test for mono) |
|
|
when do you use the viral capsid antigens (VCA) test? |
when infectious mononucleosis is suspected, but the heterophile antibody test is negative (esp in children under 10)
--> detects presence of EBV-VCA (IgM & IgG) |
|
|
In IM, when do you normally see the EBV-VCA (IgM) antibody? what does it indicate? |
during the 1st week of infection best indicator of current infection |
|
|
In IM, when do you normally see the EBV-VCA (IgG) antibody? what does it indicate? |
about 7 days after exposure indicating either current or past infection. |
|
|
In IM, when do you normally see the EBNA antibody? what does it indicate?
|
appears late in 1st month of infection and persists indefinitely; indicates past infection |
|
|
In IM, what is EBV-EA's indicative of? |
EBV-carrier state
|
|
|
what is the first test you do in someone that you suspect has infectious mononucleosis?
|
heterophil antibody test
|
|
|
If heterophile (-) & VCA (+) pt may have non-heterophile producing IM. If both heterophile (-) & VCA (-) what is the likely reason? |
pt has CMV mononucleosis
(NOT infectious mononucleosis (IM)) |
|
|
what happens when the EBV incorporates its genome into host cell DNA w/o activating the B cell? what is potential outcome of this? |
it establishes a latent infection--> transforms B-cells into immortal, constantly dividing cells (potentially causing cancer) |
|
|
what virus is associated w/ causing Burkitt's lymphoma? |
EBV--genome detected in tumor cells
(EBNA transforms B-cells into immortal lymphocytes= lymphoma) |
|
|
what is the vector for yellow fever AND dengue fever? |
mosquitos (aedes aegypti)
(live in still shallow water pools)
(both are also +ssRNA, icosahedral capsid, flavavirus family) |
|
|
where does the yellow fever virus replicate inside of the body? |
liver
|
|
|
How many phases are involved in yellow fever? what does each phase entail? |
3 phase: 1) slight fever, headache, muscle aches. (3-6 days) 2) remission, 3) delirium, seizures, coma, jaundice "yellow jack", and massive intestinal hemmorrhage "black vomit" |
|
|
how many phases are involved in dengue fever? what does each phase entail?
|
2 phases: 1) Fever, severe pain in the head and muscles "breakbone fever" ( remission - 24 hrs) 2) return of the fever and a bright red rash. |
|
|
how many strains are there of dengue virus?
|
4
|
|
|
Dengue fever is usually self-limiting UNLESS what?
|
reinfected
refinfection--> dengue hemorrhagic fever (DHF) --> internal bleeding, shock, death |
|
|
what can cause fungemia? |
complications due to venous or arterial catherization
|
|
|
what populations are most at risk for developing fungemia? |
AIDS/ immunocompromised; pts on antimicrobial therapy, radiation, antineoplastic drugs. (transplant pts, IV drug users)
(failed immune hosts) |
|
|
what type of endocarditis are IV drug users prone to? what is the most common cause? |
candida endocarditis; candida albicans |
|
|
candidemia is associated with significant ______ and _____ rates. |
morbidity, mortality (up to 75%)
*4th most common nosocomial disease
(give all pts candidemia tx) |
|
|
Which Candida sp.? MOST common- adults- pediatrics- bone marrow transplant- |
MOST common- C albicans adults- C. tropicalis & C. glabrata pediatrics- C. parapsilosis bone marrow transplant- C. krusei |
|
|
what are the four overlapping forms of invasive candidiasis? |
catheter related candidemia, acute disseminated candidiasis, chronic disseminated candidiasis, deep organ candidiasis |
|
|
what is primary infection of catheter-related candidemia? |
is on the catheter or related to the fibrin clot which forms on the catheter (focal point)
(seeding of biofilm may occur & cause hematogenous spread) |
|
|
where does acute disseminated candidiasis originate from? |
contaminated catheter
(spreads from focal point to multiple organs) |
|
|
what is another name for chronic desseminated candidiasis? when does it occur? |
hepatosplenic candidiasis;
exclusively occurs following prolonged episodes of bone marrow dysfunction and neutropenia (leukemia tx) |
|
|
What is the ONLY manifestation of deep organ candidiasis? |
focal infection of a specific organ |
|
|
name which disseminated form of fungi causes these infections: |
1) coccidioidomycosis (coccidioides immitus) |
|
|
what is the infectious phase of malaria?
|
the production of sporozoites that migrate from the gut to the salivary glands of the anopheles mosquito |
|
|
in malaria, where do the sporozoites invade inside of the human and what do they replicate into? |
liver cells; replicate many times into merozoites & infect RBCs |
|
|
what happens to the RBCs in malaria when they are invaded by merozoites? |
the merozoites continue to replicate and lyse the RBCs and invade other RBCs, some develop into m & f gametes |
|
|
what parasite causes the most severe form of malaria? what makes it the most severe? |
plasmodium falciparum; the parasite infects all erythrocytes any phase of an erythrocytic life cycle--> rigid RBC membrane |
|
|
what two parasites cause relapsing malaria? how does this happen? |
plasmodium vivax, and ovale;
after tx, tx-resistant parasites reside dormant in the liver and later multiply in an exoerythrocytic cycle eventually invading RBCs and beginning a typical erythrocytic cycle. |
|
|
what is a complication of recurrent malarial infections?
|
can cause severe anemia
|
|
|
which plasmodium produces long-lasting infections and is most often asymptomatic?
|
plasmodium malariae
|
|
|
why are the clinical manifestations of malaria delayed? |
7-30 day incubation period
also due to prophylaxis txs (delay weeks- months) |
|
|
what is a key indicator of malaria found on a peripheral blood smear?
what stains are used? |
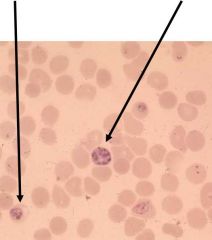
schuffner's dots- small purplish red granules found in RBCs (plasmodium vivax)
wright (img) & giemsa stain |
|
|
what are the physical findings of malaria?
what physical findings are specific to P. falciparum? |
elevated temp, weakness, enlarged spleen (due to abnormal RBCs)
|
|
|
what lab results are indicative of malaria, particularly malaria caused by P. falciparum?
|
mild anemia, thrombocytopenia, elevated bilirubin, aminotransferases (liver enzymes) albuminuria, urinary casts |
|
|
what are some complications of severe malaria? |
-cerebral malaria** (abnormal behavior, coma, seizure) -severe anemia, hemoglobinuria -pulmonary edema, -abnormal blood coags, thrombocytopenia -Cardiovascular collapse, acute kidney failure, -hypoglycemia & metabolic acidosis -hyperparasitemia (>5% RBCs infected) |
|
|
what are the four ways that you can diagnose malaria? |
microscopic blood smear w/ wright/giemsa antigen detection (malaria RDTs, rapid test) molecular diagnosis (PCR) serology (IFA, ELISA) |
|
|
Gold standard laboratory confirmation for malaria |
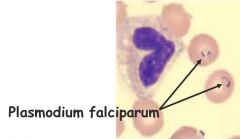
Microscopic blood smear stained w/ giemsa, wrights |
|
|
what are the four groups of individuals who have resistance to malaria? why? |
sickle-cell: erythrocyte membrane is abnormal and becomes stiff under low oxygen tension making them resistant to plasmodium sp infection |
|
|
what population generally has a lack of blood antigens, duffy A and Duffy B? |
african americans |
|
|
what do the parasites in babesiosis invade and induce? |
invade RBCs and induce a febrile dz (hemolytic anemia, hemoglobinuria, shock, death) |
|
|
what are the 2 species responsible for the majority of human infections in babesiosis? what are the 2 hosts? which one is the vector? |
responsible: Babesia microti (mouse) & divergens (cattle)
Vector: tick
|
|
|
T/F |
true |
|
|
where does babesiosis regionally occur? |
coastal areas of NE US, offshore islands of NY and massachusetts |
|
|
what are the two ways in which you can diagnose babesiosis? which one is used just as a confirmatory test? |
direct blood smears and IFA (confirmatory) |
|
|
what does a direct blood smear of babesiosis look like?
|
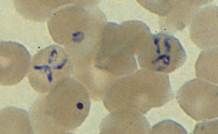
tetrad formation- parasites in RBCs. |
|
|
what other disease has the same vector as babesiosis? |
lyme |
|
|
how are individuals infected with schistosomiasis "blood flukes"? |
through contaminated water; |
|
|
what form of schistosomiasis infects humans? |
cercariae
(penetrates skin "swimmers itch" & enters venous system--> heart & portal circulation) |
|
|
which schistoma sp. has a prediliction for the bladder? which two are found in fecal matter? |
bladder: S. hematobium |
|
|
what are the organs infected by schistosomiasis? what does it cause?
|
organs: |
|
|
what is a key diagnostic find for schistosomiasis? |
eosinophilia |
|
|
what two types of schistoma cause katayama's fever (fever, cough, abdominal pain, bloody diarrhea, hepatosplenomegaly, & eosinophila)? |
S. mansoni and S. japonicum |
|
|
what type of schistoma can can CNS lesions by depositing eggs in the brain? what about in the spinal cord? |
brain: japonicum |
|
|
what are the two host immune responses to schistosomiasis?
|
IgE and eosinophil-mediated cytotoxicity
|
|
|
how do you diagnose schistosomiasis? which sp. can you find in the urine? |
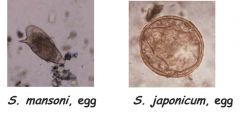
microscopy (stool and urine), and antibody detection. |
|
|
what causes chagas disease? chronic form of african sleeping sickness? acute african sleeping sickness? |
trypanosoma cruzi; trypanosoma brucei gambiense; trypanosoma brucei rhodesiense |
|
|
what does chagas's disease primarily affect?
|
NS and heart |
|
|
what can chronic infections of trypanosoma cruzi cause?
what vectors spread it? |
dementia, damage to heart muscle and death;
Triatomine (reduviid) "kissing bugs" (variety of kinds^) |
|
|
what regions can you find chagas disease?
|
central and south america
(triatomine bugs live in mud, etc that poor ppl make their home out of in these countries) |
|
|
how do kissing bugs infect human hosts? |
the bugs poop on your face (usually when sleeping) & sometimes directly into eyes. Then you rub the infected fecal material into your eyes, mouth or open cuts. Or by eating uncooked food contaminated by that fecal material. |
|
|
what sign is closely associated with the acute stage of chagas's disease? |

romana's sign: eye on one side swollen
(brain damage & death may also occur in infants & young children) |
|
|
how many stages are associated with chagas's disease? what are they? |
3; |
|
|
T/F |
false |
|
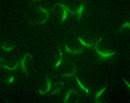
what are kinetoplastids? |
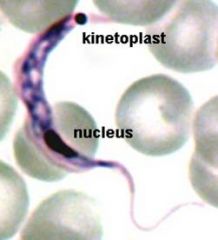
trypanosome organelles w/ mitochondrial DNA, easily immunoflouresecent labelled |
|
|
what is the life cycle of african sleeping sickness? |
Metacyclic trypomastigotes (MT) --> Long slender (LS) --> Short Stumpy (SS) --> Procyclic trypamastigoes (PT) --> epimastigotes (E)
(many types makes it hard for immune system to respond= cyclic symptoms) |
|
|
which phase of the life cycle of african sleeping sickness resides in salivary glands?
in what insect does the African sleeping sickness reside (vector)? |
metacyclic trypomastigotes--
vector= tsetse fly (Glossina) |
|
|
how do the trypomastigoes of the african sleeping sickness elude the immune system? |
via antigenic variance (long slender, short stumpy stages) |
|
|
where are procyclic trypomastigotes develop in african sleeping sickness? |
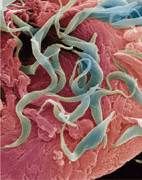
in the gut |
|
|
what is the hallmark of african sleeping sickness?
Disease progression |
invasion of the CNS-- NS impairment (crosses BBB- meningoencephalitis-fatigue during day & agitation at night-coma/death);
incubation (possible chancre)--> acute blood stage infection (fever, headache)--> lymph invasion (weight loss, fever, rash, itch)--> relapse |
|
|
why do relapses occur in african sleeping sickness? |
d/t antigenic variation of trypanosomal surface--> life cycle exhibits different morphologies |
|
|
what vector transmits leishmaniasis?
what parasite causes this? |
vector= sandflies (phlebotomus)
leshmania donovani |
|
|
where are the amastigote (kala-azar) forms of leishmaniasis found? |
in reticuloendothelial cells of the viscera (spleen, lymph nodes, liver, intestines) |
|
|
what are the sxs of leishmaniasis?
|
low grade fever, anemia, protrusion of abdomen d/t enlargement of spleen and liver, edema, bleeding mucus membranes, breathing difficulties diarrhea |
|
|
what are the possible complications of leishmaniasis? |
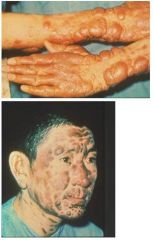
post kala-azar dermal lesihmanoid, badly disfigured face DEATH (if untreated, w/i 2-3 yrs) |
|
|
what is the diagnostic phase of the leishmaniasis life cycle? |
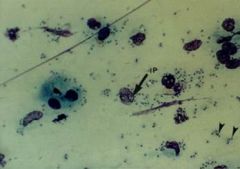
amastigotes w/i macrophages in the various organs
amastigotes = intracellular LD bodies (Leshmania donovani) |
|
|
what is the geographical distribution of visceral leishmaniasis?
|
south america (mostly), some africa and Mediterranean.
|
|
|
what is caused by infections w/ nematodes? |
filariasis (roundworms)
|
|
|
what three species are responsible for most of the morbidity due to filariasis? |
wuchereria bancrofti, (lymphatic filariasis) brugia malayi, (lymphatic filariasis) onchocerca volvulus (river blindness) |
|
|
what two parasites infiltrate the subcutaneous tissues? lymphatics? |
subQ: onchocerca volvulus, loa loa
(*onchocerca also migrate to eyes & can be seen there) |
|
|
Filarial life cycle
where do microfilariae develop into larvae? |
Mosquito bites human & ingest microfilariae--> microfilariae develop into larvae--> Mosquito bites human & deposits larvae--> larvae migrate to lymph vessels--> larvae mature in worms (filariae causing lymph blockage (elephantaiasis)
in arthropod (mosquito) = vector |
|
|
what are the clinical manifestations of lymphatic filariasis? |
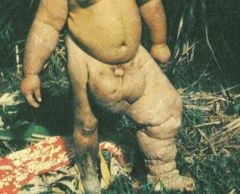
asymptomatic microfilaremia, lymphadema, elphantiasis, febrile lymphagitis & lymphadenitus |
|
|
what cell type of prominent in filarial infections?
|
eosinophils
|
|
|
what are the three diagnostic techniques that we can use for filariasis? |
-microscopic examination (most common, need periodic blood collection due to fast life-cycle or skin snips to identify microfilariae)
-antigen detection (beneficial if small amount of organism in blood stream)
-antibody detection (not very accurate) |

